Dosulepin
Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression.[4][9][10] Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom, but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs.[9][11][12] It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.[4][13][14]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prothiaden, others |
| Other names | IZ-914, KS-1596[1][2][3], dothiepin (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30%[4] |
| Protein binding | 84%[5] |
| Metabolism | Hepatic (N-demethylation, S-oxidation, glucuronidation)[5] |
| Metabolites | Northiaden, dothiepin sulfoxide, northiaden sulfoxide, glucuronide conjugates[4] |
| Elimination half-life | Dothiepin: 14.4–23.9 hours[4] Dothiepin sulfoxide: 22.7–25.5 hours[4] Northiaden: 34.7–45.7 hours[4] Northiaden sulfoxide: 24.2–33.5 hours[4] |
| Excretion | Urine: 56%[4] Feces: 15%[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.665 |
| Chemical and physical data | |
| Formula | C19H21NS |
| Molar mass | 295.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Medical uses
Dosulepin is used for the treatment of major depressive disorder.[4][5][15][16] There is clear evidence of the efficacy of dosulepin in psychogenic facial pain, though the drug may be needed for up to a year.[17]
Contraindications
Contraindications include:[5]
- Epilepsy as it can lower the seizure threshold
- TCAs should not be used concomitantly or within 14 days of treatment with monoamine oxidase inhibitors due to the risk for serotonin syndrome
- Acute recovery phase following myocardial infarction as TCAs may produce conduction defects and arrhythmias
- Liver failure
- Hypersensitivity to dosulepin
Side effects
Common adverse effects:[5]
- Drowsiness
- Extrapyramidal symptoms
- Tremor
- Disorientation
- Dizziness
- Paresthesias
- Alterations to ECG patterns
- Dry mouth
- Sweating
- Urinary retention
- Hypotension
- Postural hypotension
- Tachycardia
- Palpitations
- Arrhythmias
- Conduction defects
- Increased or decreased libido
- Nausea
- Vomiting
- Constipation
- Blurred vision
Less common adverse effects:[5]
- Disturbed concentration
- Delusions
- Hallucinations
- Anxiety
- Fatigue
- Headaches
- Restlessness
- Excitement
- Insomnia
- Hypomania
- Nightmares
- Peripheral neuropathy
- Ataxia
- Incoordination
- Seizures
- Paralytic ileus
- Hypertension
- Heart block
- Myocardial infarction
- Stroke
- Gynecomastia (swelling of breast tissue in males)
- Testicular swelling
- Impotence
- Epigastric distress
- Abdominal cramps
- Parotid swellings
- Diarrhea
- Stomatitis (swelling of the mouth)
- Black tongue
- Peculiar taste sensations
- Cholestatic jaundice
- Altered liver function
- Hepatitis (swelling of the liver)
- Skin rash
- Urticaria (hives)
- Photosensitisation
- Skin blisters
- Angioneurotic edema
- Weight loss
- Urinary frequency
- Mydriasis
- Weight gain
- Hyponatremia (low blood sodium)
- Movement disorders
- Dyspepsia (indigestion)
- Increased intraocular pressure
- Changes in blood sugar levels
- Thrombocytopenia (an abnormally low number of platelets in the blood. This makes one more susceptible to bleeds)
- Eosinophilia (an abnormally high number of eosinophils in the blood)
- Agranulocytosis (a dangerously low number of white blood cells in the blood leaving one open to potentially life-threatening infections)
- Galactorrhea (lactation that is unassociated with breastfeeding and lactation)
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other TCAs.[15] Dosulepin may be particularly toxic in overdose compared to other TCAs.[15] The onset of toxic effects is around 4–6 hours after dosulepin is ingested.[5] In order to minimise the risk of overdose it is advised that patients only receive a limited number of tablets at a time so as to limit their risk of overdosing.[5] It is also advised that patients are not prescribed any medications that are known to increase the risk of toxicity in those receiving dosulepin due to the potential for mixed overdoses.[5] The medication should also be kept out of reach of children.[5]
Interactions
Dosulepin can potentiate the effects of alcohol and at least one death has been attributed to this combination.[5] TCAs potentiate the sedative effects of barbiturates, tranquilizers and CNS depressants.[5] Guanethidine and other adrenergic neuron blocking drugs can have their antihypertensive effects blocked by dosulepin.[5] Sympathomimetics may potentiate the sympathomimetic effects of dosulepin.[5] Due to the anticholinergic and antihistamine effects of dosulepin anticholinergic and antihistamine medications may have their effects potentiated by dosulepin and hence these combinations are advised against.[5] Dosulepin may have its postural hypotensive effects potentiated by diuretics.[5] Anticonvulsants may have their efficacy reduced by dosulepin due to its ability to reduce the seizure threshold.[5]
Pharmacology
Pharmacodynamics
| Site | DSP | NTD | Species | Ref |
|---|---|---|---|---|
| SERT | 8.6–78 | 192 | Human/rat | [19][14] |
| NET | 46–70 | 25 | Human/rat | [19][14] |
| DAT | 5,310 | 2,539 | Human/rat | [19][14] |
| 5-HT1A | 4,004 | 2,623 | Rat | [20] |
| 5-HT2A | 152 | 141 | Rat | [14] |
| α1 | 419 | 950 | Rat | [14] |
| α2 | 2,400 | ND | Human | [21] |
| H1 | 3.6–4 | 25 | Human/rat | [14][21] |
| mACh | 25–26 | 110 | Human/rat | [14][22] |
| M1 | 18 | ND | Human | [23] |
| M2 | 109 | ND | Human | [23] |
| M3 | 38 | ND | Human | [23] |
| M4 | 61 | ND | Human | [23] |
| M5 | 92 | ND | Human | [23] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Dosulepin is a transporter blocker of the serotonin transporter (SERT) and the norepinephrine transporter (NET), thereby acting as an SNRI.[14][13] It is also an antagonist of the histamine H1 receptor, α1-adrenergic receptor, serotonin 5-HT2 receptors, and muscarinic acetylcholine receptors (mACh), as well as a blocker of voltage-gated sodium channels (VGSCs).[14][4] The antidepressant effects of dosulepin are thought to be due to inhibition of the reuptake of norepinephrine and possibly also of serotonin.[4]
Dosulepin has three metabolites, northiaden (desmethyldosulepin), dosulepin sulfoxide, and northiaden sulfoxide, which have longer terminal half-lives than that of dosulepin itself.[14] However, whereas northiaden has potent activity similarly to dosulepin, the two sulfoxide metabolites have dramatically reduced activity.[14] They have been described as essentially inactive, and are considered unlikely to contribute to either the therapeutic effects or side effects of dosulepin.[14] Relative to dosulepin, northiaden has reduced activity as a serotonin reuptake inhibitor, antihistamine, and anticholinergic and greater potency as a norepinephrine reuptake inhibitor,[14] similarly to other secondary amine TCAs.[24][25] Unlike the sulfoxide metabolites, northiaden is thought to play an important role in the effects of dosulepin.[14]
Although Heal & Cheetham (1992) reported relatively high Ki values of 12 and 15 nM for dosulepin and northiaden at the rat α2-adrenergic receptor and suggested that antagonism of the receptor could be involved in the antidepressant effects of dosulepin,[14] Richelson & Nelson (1984) found a low KD of only 2,400 nM for dosulepin at this receptor using human brain tissue.[21] This suggests that it in fact has low potency for this action, similarly to other TCAs.[21]
Pharmacokinetics
Dosulepin is readily absorbed from the small intestine and is extensively metabolized on first-pass through the liver into its chief active metabolite, northiaden.[5] Peak plasma concentrations of between 30.4 and 279 ng/mL (103–944 nmol/L) occur within 2–3 hours of oral administration.[5] It is distributed in breast milk and crosses the placenta and blood-brain barrier.[5] It is highly bound to plasma proteins (84%), and has a whole-body elimination half-life of 51 hours.[5]
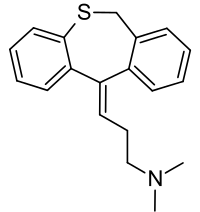
Chemistry
Dosulepin is a tricyclic compound, specifically a dibenzothiepine, and possesses three rings fused together with a side chain attached in its chemical structure.[26] It is the only TCA with a dibenzothiepine ring system to have been marketed.[26][27] The drug is a tertiary amine TCA, with its side chain-demethylated metabolite northiaden (desmethyldosulepin) being a secondary amine.[28][29] Other tertiary amine TCAs include amitriptyline, imipramine, clomipramine, doxepin, and trimipramine.[30][31] Dosulepin exhibits (E) and (Z) stereoisomerism like doxepin but in contrast the pure E or trans isomer is used medicinally.[1][13][32] The drug is used commercially as the hydrochloride salt; the free base is not used.
History
Dosulepin was developed by SPOFA.[33] It was patented in 1962 and first appeared in the literature in 1962.[33] The drug was first introduced for medical use in 1969, in the United Kingdom.[33][34]
Society and culture
Generic names
Dosulepin is the English and German generic name of the drug and its INN and BAN, while dosulepin hydrochloride is its BANM and JAN.[1][2][35][3] Dothiepin is the former BAN of the drug while dothiepin hydrochloride is the former BANM and remains the current USAN.[1][2][35][3] Its generic name in Spanish and Italian and its DCIT are dosulepina, in French and its DCF are dosulépine, and in Latin is dosulepinum.[2][3]
Brand names
Dosulepin is marketed throughout the world mainly under the brand name Prothiaden.[2][3] It is or has been marketed under a variety of other brand names as well, including Altapin, Depresym, Dopress, Dothapax, Dothep, Idom, Prepadine, Protiaden, Protiadene, Thaden, and Xerenal.[1][35][2][3]
Availability
Dosulepin is marketed throughout Europe (as Prothiaden, Protiaden, and Protiadene), Australia (as Dothep and Prothiaden), New Zealand (as Dopress) and South Africa (as Thaden).[2][3][10][15][16] It is also available in Japan, Hong Kong, Taiwan, India, Singapore, and Malaysia.[2][3][10] The drug is not available in the United States or Canada.[2][3][10]
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 468–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 369–. ISBN 978-3-88763-075-1.
- https://www.drugs.com/international/dosulepin.html
- Lancaster SG, Gonzalez JP (1989). "Dothiepin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness". Drugs. 38 (1): 123–47. doi:10.2165/00003495-198938010-00005. PMID 2670509.
- "Dothep Dothiepin hydrochloride" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 1 November 2013. Retrieved 3 December 2013.
- https://chem.nlm.nih.gov/chemidplus/rn/25627-36-5
- https://chem.nlm.nih.gov/chemidplus/rn/113-53-1
- https://chem.nlm.nih.gov/chemidplus/rn/897-15-4
- Donovan S, Dearden L, Richardson L (1994). "The tolerability of dothiepin: a review of clinical studies between 1963 and 1990 in over 13,000 depressed patients". Prog. Neuropsychopharmacol. Biol. Psychiatry. 18 (7): 1143–62. doi:10.1016/0278-5846(94)90117-1. PMID 7846285.
- Dosulepin Hydrochloride. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 5 December 2011. Retrieved 15 August 2017.
- Thanacoody HK, Thomas SH (2005). "Tricyclic antidepressant poisoning : cardiovascular toxicity". Toxicol Rev. 24 (3): 205–14. doi:10.2165/00139709-200524030-00013. PMID 16390222.
- Gillman PK (2007). "Tricyclic antidepressant pharmacology and therapeutic drug interactions updated". Br. J. Pharmacol. 151 (6): 737–48. doi:10.1038/sj.bjp.0707253. PMC 2014120. PMID 17471183.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 607–. ISBN 978-1-60913-345-0.
- Heal, David; Cheetham, Sharon; Martin, Keith; Browning, John; Luscombe, Graham; Buckett, Roger (1992). "Comparative pharmacology of dothiepin, its metabolites, and other antidepressant drugs". Drug Development Research. 27 (2): 121–135. doi:10.1002/ddr.430270205. ISSN 0272-4391.
- Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- C Feinmann; M Harris; R Cawley (11 February 1984). "Psychogenic facial pain: presentation and treatment". Br Med J (Clin Res Ed). 288 (6415): 436–8. doi:10.1136/bmj.288.6415.436. PMC 1444752. PMID 6419955.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- Sánchez C, Hyttel J (1999). "Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding". Cell. Mol. Neurobiol. 19 (4): 467–89. doi:10.1023/A:1006986824213. PMID 10379421.
- Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- Cusack B, Nelson A, Richelson E (1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217.
- Stanton T, Bolden-Watson C, Cusack B, Richelson E (1993). "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochem. Pharmacol. 45 (11): 2352–4. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- Robert E. Hales; Stuart C. Yudofsky; Glen O. Gabbard (2011). Essentials of Psychiatry. American Psychiatric Pub. pp. 468–. ISBN 978-1-58562-933-6.
- Carl A. Burtis; Edward R. Ashwood; David E. Bruns (14 October 2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics - E-Book. Elsevier Health Sciences. pp. 1129–. ISBN 978-1-4557-5942-2.
- J. K. Aronson (2009). Meyler's Side Effects of Psychiatric Drugs. Elsevier. pp. 7–. ISBN 978-0-444-53266-4.
- Michael S Ritsner (15 February 2013). Polypharmacy in Psychiatry Practice, Volume I: Multiple Medication Use Strategies. Springer Science & Business Media. pp. 270–271. ISBN 978-94-007-5805-6.
- Neal R. Cutler; John J. Sramek; Prem K. Narang (20 September 1994). Pharmacodynamics and Drug Development: Perspectives in Clinical Pharmacology. John Wiley & Sons. pp. 160–. ISBN 978-0-471-95052-3.
- Pavel Anzenbacher; Ulrich M. Zanger (23 February 2012). Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. pp. 302–. ISBN 978-3-527-64632-6.
- Patricia K. Anthony (2002). Pharmacology Secrets. Elsevier Health Sciences. pp. 39–. ISBN 1-56053-470-2.
- Philip Cowen; Paul Harrison; Tom Burns (9 August 2012). Shorter Oxford Textbook of Psychiatry. OUP Oxford. pp. 532–. ISBN 978-0-19-162675-3.
- Psychotropic Agents: Part I: Antipsychotics and Antidepressants. Springer Science & Business Media. 6 December 2012. pp. 354–. ISBN 978-3-642-67538-6.
- Andersen J, Kristensen AS, Bang-Andersen B, Strømgaard K (2009). "Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters". Chem. Commun. (25): 3677–92. doi:10.1039/b903035m. PMID 19557250.
- Richard C. Dart (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 836–. ISBN 978-0-7817-2845-4.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 105–. ISBN 978-94-011-4439-1.