Methysergide
Methysergide (1-methyl-D-lysergic acid butanolamide or UML-491) also known as methysergide maleate, is an ergot derived prescription drug used for the prophylaxis of difficult to treat migraine and cluster headaches.
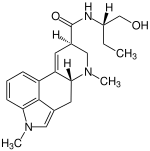 | |
| Clinical data | |
|---|---|
| Trade names | Deseril, Sansert |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a603022 |
| Pregnancy category | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.041 |
| Chemical and physical data | |
| Formula | C21H27N3O2 |
| Molar mass | 353.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methysergide is no longer recommended as a first line treatment protocol by international headache societies, hospitals, and neurologists in private practice, for migraines or cluster headaches as side effects were first reported with long-term use in the late 1960s, and ergot based treatments fell out of favor for the treatment of migraines with the introduction of triptans in the 1980s.
Medical uses
Methysergide is used exclusively to treat episodic and chronic migraine and for episodic and chronic cluster headaches.[1] Methysergide is one of the most effective[2] medications for the prevention of migraine, but is not intended for the treatment of an acute attack, it is to be taken daily as a preventative medication.
Efficacy
Methysergide has been known as an effective treatment for migraine and cluster headache for over 50 years. A 2016 investigation by the European Medicines Agency due to long-held questions about safety concerns was performed. To assess the need for continuing availability of methysergide, the International Headache Society performed an electronic survey among their professional members.
The survey revealed that 71.3% of all respondents had ever prescribed methysergide and 79.8% would prescribe it if it were to become available. Respondents used it more in cluster headache than migraine, and reserved it for use in refractory patients.
The European Medicines Agency concluded "that the vast majority of headache experts in this survey regarded methysergide a unique treatment option for specific populations for which there are no alternatives, with an urgent need to continue its availability."
This position was supported by the International Headache Society.[3]
Other uses
It is also used in carcinoid syndrome to treat severe diarrhea.[1] It may also be used in the treatment of serotonin syndrome.[4]
Side effects
It has a known side effect, retroperitoneal fibrosis/retropulmonary fibrosis,[5] which is severe, although uncommon. This side effect has been estimated to occur in 1/5000 patients.[6] In addition, there is an increased risk of left-sided cardiac valve dysfunction.[2]
Pharmacology
Methysergide interacts with serotonin (5-HT) receptors. Its therapeutic effect in migraine prophylaxis has been associated with its antagonism at the 5-HT2B receptor.[7]
It is an antagonist at the 5-HT2C receptor, while at the 5-HT1A receptor it serves as a partial agonist.[8][9][10] It is known to have partial agonist effects on some of the other 5-HT receptors as well.[11] Methysergide is metabolised into methylergometrine in humans, which is responsible for its psychedelic effects.[12]
It antagonizes the effects of serotonin in blood vessels and gastrointestinal smooth muscle, but has few of the properties of other ergot alkaloids.[13]
History
Harold Wolff's theory of vasodilation in migraine is well-known. Less known is his search for a perivascular factor that would damage local tissues and increase pain sensitivity during migraine attacks. Serotonin was found to be among the candidate agents to be included.
In the same period, serotonin was isolated (1948) and, because of its actions, an anti-serotonin drug was needed.
Methysergide was synthesized from lysergic acid by adding a methyl group and a butanolamid group. This resulted in a compound with selectivity and high potency as a serotonin (5-HT) inhibitor. Based on the possible involvement of serotonin in migraine attacks, it was introduced in 1959 by Sicuteri as a preventive drug for migraine. The clinical effect was often excellent, but 5 years later it was found to cause retroperitoneal fibrosis after chronic intake.
Consequently, the use of the drug in migraine declined considerably, but it was still used as a 5-HT antagonist in experimental studies. In 1974 Saxena showed that methysergide had a selective vasoconstrictor effect in the carotid bed and in 1984 he found an atypical receptor. This finding provided an incentive for the development of sumatriptan.[14]
Novartis withdrew it from the U.S. market after taking over Sandoz, but currently lists it as a discontinued product.[15]
Production and availability
US production of Methysergide, (Sansert), was discontinued on the manufacturer's own behalf in 2002. Sansert had previously been produced by Sandoz, which merged with Ciba-Geigy in 1996, and led to the creation of Novartis. In 2003 Novartis united its global generics businesses under a single global brand, with the Sandoz name and product line reviewed and reestablished.
Controversy
Methysergide has been an effective treatment for migraine and cluster headache for over 50 years but has systematically been suppressed from the migraine and cluster headache marketplace for over 15 years due to unqualified risk benefit/ratio safety concerns.[16]
Many cite the potential side effects of retroperitoneal/retropulmonary fibrosis as the prime reason methysergide is no longer frequently prescribed, but retroperitoneal fibrosis, and retropulmonary fibrosis, were documented as side effects as early as 1966,[17] and 1967,[18] respectively.
Updated guidelines published by Britain's NHS Migraine Trust in 2014 recommended "Methysergide medicines are now only to be used for preventing severe intractable migraine and cluster headache when standard medicines have failed".[19]
See also
- Amesergide
- CGRP
- CGRP receptor antagonist
- Ergot
- Triptan
References
- http://tranquilene.com/methysergide.html
- Joseph T, Tam SK, Kamat BR, Mangion JR (2003). "Successful repair of aortic and mitral incompetence induced by methylsergide maleate: confirmation by intraoperative transesophageal echocardiography". Echocardiography. 20 (3): 283–7. doi:10.1046/j.1540-8175.2003.03027.x. PMID 12848667.
- MacGregor, E. Anne; Evers, Stefan; International Headache Society (2016-07-21). "The role of methysergide in migraine and cluster headache treatment worldwide - A survey in members of the International Headache Society". Cephalalgia: An International Journal of Headache. 37 (11): 1106–1108. doi:10.1177/0333102416660551. ISSN 1468-2982. PMID 27449673.
- Sporer, KA (1995). "The Serotonin Syndrome Implicated Drugs, Pathophysiology and Management". Drug Safety. 13 (2): 94–104. doi:10.2165/00002018-199513020-00004. PMID 7576268.
- "Retroperitoneal Fibrosis Imaging: Overview, Radiography, Computed Tomography". 30 March 2017 – via eMedicine. Cite journal requires
|journal=(help) - Silberstein, SD (1 Sep 1998). "Methysergide". Cephalalgia. 18 (7): 421–35. doi:10.1046/j.1468-2982.1998.1807421.x. PMID 9793694.
- Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H (May 1996). "Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache?". Eur. J. Neurosci. 8 (5): 959–67. doi:10.1111/j.1460-9568.1996.tb01583.x. PMID 8743744.
- Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-07145-4. Page 187
- Saxena PR, Lawang A (October 1985). "A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors". Arch Int Pharmacodyn Ther. 277 (2): 235–52. PMID 2933009.
- Pubchem. "Methysergide". pubchem.ncbi.nlm.nih.gov.
- Colpaert FC, Niemegeers CJ, Janssen PA (October 1979). "In vivo evidence of partial agonist activity exerted by purported 5-hydroxytryptamine antagonists". Eur. J. Pharmacol. 58 (4): 505–9. doi:10.1016/0014-2999(79)90326-1. PMID 510385.
- Bredberg, U.; Eyjolfsdottir, G. S.; Paalzow, L.; Tfelt-Hansen, P.; Tfelt-Hansen, V. (1 January 1986). "Pharmacokinetics of methysergide and its metabolite methylergometrine in man". European Journal of Clinical Pharmacology. 30 (1): 75–77. doi:10.1007/BF00614199. PMID 3709634.
- Pubchem. "methysergide". pubchem.ncbi.nlm.nih.gov. Retrieved 2017-09-06.
- Koehler, P. J.; Tfelt-Hansen, P. C. (November 2008). "History of methysergide in migraine". Cephalalgia: An International Journal of Headache. 28 (11): 1126–1135. doi:10.1111/j.1468-2982.2008.01648.x. ISSN 1468-2982. PMID 18644039.
- "Sansert / methysergide maleate FDA New drug application 012516 international drug patent coverage, generic alternatives and suppliers". Deep knowledge on small-molecule drugs and the global patents covering them. Retrieved 2017-09-06.
- "CHMP referral assessment report" (PDF).
- 1966 Webb, John A. (30 Apr 1966). "Methysergide and Retroperitoneal Fibrosis". British Medical Journal. 1 (5495): 1100. doi:10.1136/bmj.1.5495.1110-a. PMC 1844020. PMID 20790951.
- Graham, Jr (17 Feb 1966). "Fibrotic disorders associated with methysergide therapy for headache". N Engl J Med. 274 (7): 359–68. doi:10.1056/NEJM196602172740701. PMID 5903120.
- "Methysergide - The Migraine Trust". The Migraine Trust. Retrieved 2017-09-06.
External links
- Novartis Sansert site.
- Novartis Sansert product description.
- Migraines.org More detailed information on methysergide.
- neurologychannel.com, general information on migraines.
- History of methysergide in migraine.