Mifepristone
Mifepristone, also known as RU-486, is a medication typically used in combination with misoprostol to bring about an abortion during pregnancy.[1] This combination is 97% effective during the first 63 days of pregnancy.[2] It is also effective in the second trimester of pregnancy.[3][4] Effectiveness should be verified two weeks after use.[1] It is taken by mouth.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mifegyne, Mifeprex, others |
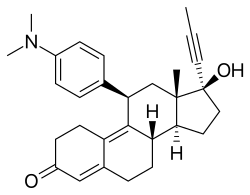
| Other names | RU-486; RU-38486; ZK-98296; 11β-[p-(Dimethylamino)phenyl]-17α-(1-propynyl)estra-4,9-dien-17β-ol-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600042 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antiprogestogen; Antiglucocorticoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 69% |
| Protein binding | 98% |
| Metabolism | Liver |
| Excretion | Feces: 83% Urine: 9% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.127.911 |
| Chemical and physical data | |
| Formula | C29H35NO2 |
| Molar mass | 429.604 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.189 g/cm3 |
| Melting point | 194 °C (381 °F) |
| Boiling point | 629 °C (1,164 °F) |
| |
| |
| (verify) | |
Common side effects include abdominal pain, feeling tired, and vaginal bleeding.[1] Serious side effects may include heavy vaginal bleeding, bacterial infection, and birth defects if the pregnancy does not end.[1] If used, appropriate follow up care needs to be available.[1][5] Mifepristone is an antiprogestogen.[1] It works by blocking the effects of progesterone, making the cervix easier to open, and promoting contraction of the uterus when exposed to misoprostol.[1]
Mifepristone was developed in 1980 and came into use in France in 1987.[6] It became available in the United States in 2000.[3] It is on the World Health Organization's List of Essential Medicines.[7] Mifepristone was approved by Health Canada in 2015 and became available in Canada in January 2017.[8] Cost and availability limits access in many parts of the developing world.[9][10]
Medical uses
Abortion
Mifepristone followed by a prostaglandin analog (misoprostol or gemeprost) is used for medical abortion.[11][12] Medical organizations have found this combination to be safe and effective. Guidelines from the Royal College of Obstetricians and Gynaecologists describe medical abortion using mifepristone and misoprostol as effective and appropriate at any gestational age.[13] The World Health Organization and the American Congress of Obstetricians and Gynecologists recommend mifepristone followed by misoprostol for first- and second-trimester medical abortion.[14][15][16][17] Mifepristone alone is less effective, resulting in abortion within 1–2 weeks in 8% to 46% of pregnancies.[18]
Cushing's syndrome
Mifepristone is used for the medical treatment of high blood sugar caused by high cortisol levels in the blood (hypercortisolism) in adults with endogenous Cushing's syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or cannot have surgery.[19][20]
Side effects
Serious complications with mifepristone are rare with about 0.04%-0.9% requiring hospitalization and 0.05% requiring blood transfusion.[26]
Nearly all women using the mifepristone/misoprostol regimen experienced abdominal pain, uterine cramping, and vaginal bleeding or spotting for an average of 9–16 days. For most women, the most severe cramps after use of misoprostol last for less than 6 hours and can generally be managed with ibuprofen.[27] Up to 8% of women experienced some type of bleeding for 30 days or more. Other less common side effects included nausea, vomiting, diarrhea, dizziness, fatigue, and fever.[28] Pelvic inflammatory disease is a very rare but serious complication.[29] Excessive bleeding and incomplete termination of a pregnancy require further intervention by a doctor (such as a repeat dose of misoprostol or a vacuum aspiration). Mifepristone is contraindicated in the presence of adrenal failure, long-term oral corticosteroid therapy (although inhaled and topical steroids are fine), hemorrhagic disorders, inherited porphyria, and hemophilia or anticoagulant use.[28] Women with an intrauterine device in their uterus should remove the IUD prior to medication abortion to avoid unnecessary cramping. Mifepristone is not effective in treating ectopic pregnancy,
A postmarketing summary found, of about 1.52 million women who had received mifepristone until April 2011 in the United States, 14 were reported to have died after application. Eight of these cases were associated with sepsis; the other six had various causes such as drug abuse and suspected murder. Other incidents reported to the FDA included 612 nonlethal hospitalizations, 339 blood transfusions, 48 severe infections, and 2,207 (0.15%) adverse events altogether.[30]
Cancer
No long-term studies to evaluate the carcinogenic potential of mifepristone have been performed. This is in accord with ICH guidelines, which do not require carcinogenicity testing in nongenotoxic drugs intended for administration for less than six months.[31]
Pregnancy
Mifepristone alone results in abortion within 1–2 weeks in 8% to 46% of pregnancies.[18][32] The effectiveness increases to greater than 90% if misoprostol is given after the mifepristone.[33] There is no evidence that the effects of mifepristone can be reversed,[18][34] although some anti-abortion groups claim that it can be reversed by giving progesterone.[35][36] Researchers in the United States initiated a trial of the so-called "reversal" regimen in 2019, but stopped prematurely due to serious safety concerns about using mifepristone without follow-up misoprostol.[37][38] Giving progesterone has not been shown to halt medication abortion, and not completing the combination regimen of mifepristone and misoprostol may cause serious bleeding.[38]
In those who continue pregnancy after use of mifepristone together with misoprostol for termination, birth defects may occur.[5] Exposure to a single large dose of mifepristone in newborn rats was not associated with any reproductive problems, although chronic low-dose exposure of newborn rats to mifepristone was associated with structural and functional reproductive abnormalities.[28] Studies in mice, rats, and rabbits revealed teratogenicity for rabbits, but not rats or mice.[28]
Pharmacology
Pharmacodynamics
Mifepristone is a steroidal antiprogestogen (IC50 = 0.025 nM for the PR), as well as an antiglucocorticoid (IC50 = 2.2 nM for the GR) and antiandrogen (IC50 = 10 nM for the AR) to a much lesser extent.[39] It antagonizes cortisol action competitively at the receptor level.[40]
In the presence of progesterone, mifepristone acts as a competitive progesterone receptor antagonist (in the absence of progesterone, mifepristone acts as a partial agonist). Mifepristone is a 19-nor steroid with a bulky p-(dimethylamino) phenyl substituent above the plane of the molecule at the 11β-position responsible for inducing or stabilizing an inactive receptor conformation and a hydrophobic 1-propynyl substituent below the plane of the molecule at the 17α-position that increases its progesterone receptor binding affinity.[41][42][43]
In addition to being an antiprogestogen, mifepristone is also an antiglucocorticoid and a weak antiandrogen. Mifepristone's relative binding affinity at the progesterone receptor is more than twice that of progesterone, its relative binding affinity at the glucocorticoid receptor is more than three times that of dexamethasone and more than ten times that of cortisol; its relative binding affinity at the androgen receptor is less than one-third that of testosterone. It does not bind to the estrogen receptor or the mineralocorticoid receptor.[44]
Mifepristone as a regular contraceptive at 2 mg daily prevents ovulation (1 mg daily does not). A single preovulatory 10-mg dose of mifepristone delays ovulation by three to four days and is as effective an emergency contraceptive as a single 1.5-mg dose of the progestin levonorgestrel.[45]
In women, mifepristone at doses greater or equal to 1 mg/kg antagonizes the endometrial and myometrial effects of progesterone. In humans, an antiglucocorticoid effect of mifepristone is manifested at doses greater or equal to 4.5 mg/kg by a compensatory increase in ACTH and cortisol. In animals, a weak antiandrogenic effect is seen with prolonged administration of very high doses of 10 to 100 mg/kg.[46][47]
In medical abortion regimens, mifepristone blockade of progesterone receptors directly causes endometrial decidual degeneration, cervical softening and dilatation, release of endogenous prostaglandins, and an increase in the sensitivity of the myometrium to the contractile effects of prostaglandins. Mifepristone-induced decidual breakdown indirectly leads to trophoblast detachment, resulting in decreased syncytiotrophoblast production of hCG, which in turn causes decreased production of progesterone by the corpus luteum (pregnancy is dependent on progesterone production by the corpus luteum through the first nine weeks of gestation—until placental progesterone production has increased enough to take the place of corpus luteum progesterone production). When followed sequentially by a prostaglandin, mifepristone 200 mg is (100 mg may be, but 50 mg is not) as effective as 600 mg in producing a medical abortion.[41][43]
'Contragestion' is a term promoted by Étienne-Émile Baulieu in the context of his advocacy of mifepristone, defining it as inclusive of some hypothesized mechanisms of action of some contraceptives and those of mifepristone to induce abortion.[48] Baulieu's definition of a 'contragestive' included any birth control method that could possibly act after fertilization and before nine-weeks gestational age.[48]
Pharmacokinetics
The elimination half-life is complex; according to the label: "After a distribution phase, elimination is at first slow, the concentration decreasing by a half between about 12 and 72 hours, and then more rapid, giving an elimination half-life of 18 hours. With radio receptor assay techniques, the terminal half-life is of up to 90 hours, including all metabolites of mifepristone able to bind to progesterone receptors."[11] Metapristone is the major metabolite of mifepristone.[49][50][51]
Chemistry
Mifepristone, also known as 11β-(4-(dimethylamino)phenyl)-17α-(1-propynyl)estra-4,9-dien-17β-ol-3-one, is a synthetic estrane steroid and a derivative of steroid hormones like progesterone, cortisol, and testosterone.[40] It has substitutions at the C11β and C17α positions and double bonds at the C4(5) and C9(10) positions.[40]
History
In April 1980, as part of a formal research project at the French pharmaceutical company Roussel-Uclaf for the development of glucocorticoid receptor antagonists, chemist Georges Teutsch synthesized mifepristone (RU-38486, the 38,486th compound synthesized by Roussel-Uclaf from 1949 to 1980; shortened to RU-486), which was discovered to also be a progesterone receptor antagonist.[52][53] In October 1981, endocrinologist Étienne-Émile Baulieu, a consultant to Roussel-Uclaf, arranged tests of its use for medical abortion in 11 women in Switzerland by gynecologist Walter Herrmann at the University of Geneva's Cantonal Hospital, with successful results announced on April 19, 1982.[52][54] On October 9, 1987, following worldwide clinical trials in 20,000 women of mifepristone with a prostaglandin analogue (initially sulprostone or gemeprost, later misoprostol) for medical abortion, Roussel-Uclaf sought approval in France for their use for medical abortion, with approval announced on September 23, 1988.[52][55]
On October 21, 1988, in response to antiabortion protests and concerns of majority (54.5%) owner Hoechst AG of Germany, Roussel-Uclaf's executives and board of directors voted 16 to 4 to stop distribution of mifepristone, which they announced on October 26, 1988.[52][56] Two days later, the French government ordered Roussel-Uclaf to distribute mifepristone in the interests of public health.[52][57] French Health Minister Claude Évin explained: "I could not permit the abortion debate to deprive women of a product that represents medical progress. From the moment Government approval for the drug was granted, RU-486 became the moral property of women, not just the property of a drug company."[52] Following use by 34,000 women in France from April 1988 to February 1990 of mifepristone distributed free of charge, Roussel-Uclaf began selling Mifegyne (mifepristone) to hospitals in France in February 1990 at a price (negotiated with the French government) of US$48 (equivalent to $93.93 in 2019) per 600-mg dose.[52]
Mifegyne was subsequently approved in Great Britain on July 1, 1991,[58] and in Sweden in September 1992,[59] but until his retirement in late April 1994, Hoechst AG chairman Wolfgang Hilger, a devout Roman Catholic, blocked any further expansion in availability.[52][60] On May 16, 1994, Roussel-Uclaf announced it was donating without remuneration all rights for medical uses of mifepristone in the United States to the Population Council,[61] which subsequently licensed mifepristone to Danco Laboratories, a new single-product company immune to antiabortion boycotts, which won FDA approval as Mifeprex on September 28, 2000.[62]
On April 8, 1997, after buying the remaining 43.5% of Roussel-Uclaf stock in early 1997,[63] Hoechst AG (US$30 (equivalent to $48.91 in 2019) billion annual revenue) announced the end of its manufacture and sale of Mifegyne (US$3.44 (equivalent to $5.61 in 2019) million annual revenue) and the transfer of all rights for medical uses of mifepristone outside of the United States to Exelgyn S.A., a new single-product company immune to antiabortion boycotts, whose CEO was former Roussel-Uclaf CEO Édouard Sakiz.[64] In 1999, Exelgyn won approval of Mifegyne in 11 additional countries, and in 28 more countries over the following decade.[65]
Society and culture
Mifepristone is on the World Health Organization's List of Essential Medicines.[7] Since 2019 it has been included on the core list, with misoprostol, with a special note "where permitted under national law and where culturally acceptable".[7]
Frequency of use
United States
Medical abortions voluntarily reported by 33 U.S. states[66] to the Centers for Disease Control and Prevention (CDC) have increased as a percentage of total abortions every year since the approval of mifepristone: 1.0% in 2000, 2.9% in 2001, 5.2% in 2002, 7.9% in 2003, 9.3% in 2004, 9.9% in 2005, 10.6% in 2006, and 13.1% in 2007 (20.3% of those at less than 9 weeks gestation).[67]
A Guttmacher Institute survey of abortion providers estimated that medical abortions accounted for 17% of all abortions and slightly over 25% of abortions before 9 weeks gestation in the United States in 2008 (94% of nonhospital medical abortions used mifepristone and misoprostol, 6% used methotrexate and misoprostol).[68] Medical abortions accounted for 32% of first trimester abortions at Planned Parenthood clinics in the United States in 2008.[69]
In 2014, an estimated 272,400 medication abortions were provided in nonhospital facilities, representing a 14% increase since 2011. Medical abortions accounted for 31% of all non-hospital abortions, compared with 24% in 2011. Half or more of all abortions (50–68%) provided by facilities with annual caseloads of fewer than 400 procedures were early medication abortions.[70]
Europe
In France, the percentage of medical abortions of all abortions continues to increase: 38% in 2003, 42% in 2004, 44% in 2005, 46% in 2006, 49% in 2007 (vs. 18% in 1996).[71] In England and Wales, 52% of early abortions (less than 9 weeks gestation) in 2009 were medical; the percentage of all abortions that are medical has increased every year for the past 14 years (from 5% in 1995 to 40% in 2009) and has more than doubled in the last five years.[72] In Scotland, 81.2% of early abortions in 2009 were medical (up from 55.8% in 1992 when medical abortion was introduced); the percentage of all abortions that are medical has increased every year for the past 17 years (from 16.4% in 1992 to 69.9% in 2009).[73] In Sweden, 85.6% of early abortions and 73.2% of abortions before the end of the 12th week of gestation in 2009 were medical; 68.2% of all abortions in 2009 were medical.[74] In Great Britain and Sweden, mifepristone is licensed for use with vaginal gemeprost or oral misoprostol. As of 2000, more than 620,000 women in Europe had had medical abortions using a mifepristone regimen.[75] In Denmark, mifepristone was used in between 3,000 and 4,000 of just over 15,000 abortions in 2005.[76]
Legal status
United States
Mifepristone was approved for abortion in the United States by the FDA in September 2000.[77] It is legal and available in all 50 states, Washington, D.C., Guam, and Puerto Rico.[78] It is a prescription drug, but it is not available to the public through pharmacies; its distribution is restricted to specially qualified licensed physicians, sold by Danco Laboratories under the trade name Mifeprex.
Roussel Uclaf did not seek U.S. approval, so in the United States legal availability was not initially possible.[79] The United States banned importation of mifepristone for personal use in 1989, a decision supported by Roussel Uclaf. In 1994, Roussel Uclaf gave the U.S. drug rights to the Population Council in exchange for immunity from any product liability claims.[61][80] The Population Council sponsored clinical trials in the United States.[81] The drug went on approvable status from 1996. Production was intended to begin through the Danco Group in 1996, but they withdrew briefly in 1997 due to a corrupt business partner, delaying availability again.[82][83]
In 2016, the US Food and Drug Administration approved mifepristone, to end a pregnancy through 70 days gestation (70 days or less since the first day of a woman's last menstrual period). The approved dosing regimen is 200 mg of mifepristone taken by mouth (swallowed). 24 to 48 hours after taking mifepristone, 800 mcg (micrograms) of misoprostol is taken buccally (in the cheek pouch), at a location appropriate for the patient.[12][84][85][86]
Mifepristone tablets have a marketing authorization in the United States for the treatment of high blood sugar caused by high cortisol levels in the blood (hypercortisolism) in adults with endogenous Cushing's syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or cannot have surgery.[19]
In 2019, the first generic form of mifepristone in the United States became available, manufactured by GenBioPro.[87]
Subsection H
Some drugs are approved by the FDA under subsection H, which has two subparts. The first sets forth ways to rush experimental drugs, such as aggressive HIV and cancer treatments, to market when speedy approval is deemed vital to the health of potential patients. The second part of subsection H applies to drugs that not only must meet restrictions for use due to safety requirements, but also are required to meet postmarketing surveillance to establish that the safety results shown in clinical trials are seconded by use in a much wider population. Mifepristone was approved under the second part of subsection H. The result is that women cannot pick the drug up at a pharmacy, but must now receive it directly from a doctor. Due to the possibility of adverse reactions such as excessive bleeding, which may require a blood transfusion, and incomplete abortion, which may require surgical intervention, the drug is only considered safe if a physician who is capable of administering a blood transfusion or a surgical abortion is available to the patient in the event of such emergencies.[88] The approval of mifepristone under subsection H included a black box warning.
Europe
Outside the United States, it is marketed and distributed by Exelgyn Laboratories under the tradename Mifegyne. Mifepristone was approved for use in France in 1988 (initial marketing in 1989), the United Kingdom in 1991, Sweden in 1992, then Austria, Belgium, Denmark, Finland, Germany, Greece, Luxembourg, the Netherlands, Spain, and Switzerland in 1999.[89] In 2000, it was approved in Norway, Russia and Ukraine. Serbia and Montenegro approved it in 2001,[90] Belarus and Latvia in 2002, Estonia in 2003, Moldova in 2004, Albania and Hungary in 2005, Portugal in 2007, Romania in 2008,[65] Bulgaria, Czech Republic and Slovenia in 2013.[91] In Italy, clinical trials have been constrained by protocols requiring women be hospitalized for three days, but the drug was finally approved on July 30, 2009 (officialized later in the year), despite strong opposition from the Vatican. In Italy, the pill must be prescribed and used in a clinical structure and is not sold at chemists.[92] It was approved in Hungary in 2005, but as of 2005 had not been released on the market yet, and was the target of protests.[93] Mifepristone is not approved in Ireland, where abortion remains illegal pending implementation of a constitutional amendment, or Poland, where abortion is highly restricted.[94]
Mifepristone 200 mg tablets (Mifegyne, Mifepristone Linepharma, Medabon) have marketing authorizations in the European Economic Area from the European Medicines Agency (EMA) for:[11][95][96]
- Early first trimester medical abortion when followed by a prostaglandin analog (misoprostol or gemeprost) through 63 days gestational age
- Second trimester medical abortion when followed by a prostaglandin analog
- Cervical softening and dilation prior to first trimester surgical abortion
- Induction of labor after fetal death in utero when prostaglandin analogs and oxytocin are contraindicated
Other countries
Mifepristone was banned in Australia in 1996. In late 2005, a private member's bill was introduced to the Australian Senate to lift the ban and transfer the power of approval to the Therapeutic Goods Administration. The move caused much debate in the Australian media and amongst politicians. The bill passed the Senate on 10 February 2006, and mifepristone is now legal in Australia. It is provided regularly at several specialized abortion clinics per state.[97][98] Mifepristone 200 mg tablets have marketing authorizations in Australia from the Therapeutic Goods Administration (TPA) for early first trimester medical abortion when followed by the prostaglandin analog misoprostol through 63 days gestational age[99] and second trimester medical abortion when followed by a prostaglandin analog.[100]
In New Zealand, pro-abortion rights doctors established an import company, Istar, and submitted a request for approval to MedSafe, the New Zealand pharmaceutical regulatory agency. After a court case brought by Right to Life New Zealand failed, use of mifepristone was permitted.[101]
The drug was approved in Israel in 1999.[102]
Clinical trials of mifepristone in China began in 1985. In October 1988, China became the first country in the world to approve mifepristone. Chinese organizations tried to purchase mifepristone from Roussel Uclaf, which refused to sell it to them, so in 1992 China began its own domestic production of mifepristone. In 2000, the cost of medical abortion with mifepristone was higher than surgical abortion and the percentage of medical abortions varied greatly, ranging from 30% to 70% in cities to being almost nonexistent in rural areas.[103][104] A report from the United States Embassy in Beijing in 2000 said mifepristone had been widely used in Chinese cities for about two years, and that according to press reports, a black market had developed with many women starting to buy it illegally (without a prescription) from private clinics and drugstores for about US$15 (equivalent to $22.27 in 2019), causing Chinese authorities to worry about medical complications from use without physician supervision.[105]
In 2001, mifepristone was approved in Taiwan.[106] Vietnam included mifepristone in the National Reproductive Health program in 2002.[107]
It is approved in only one sub-Saharan African country—South Africa, where it was approved in 2001.[108] It is also approved in one north African country—Tunisia, also in 2001.[109]
Mifepristone was approved for use in India in 2002, where medical abortion is referred to as "medical termination of pregnancy". It is only available under medical supervision, not by prescription, due to adverse reactions such as excessive bleeding, and criminal penalties are given for buying or selling it on the black market or over-the-counter at pharmacies.[110]
Medical abortion used to be available in Canada but on a limited basis using methotrexate and misoprostol. Clinical trials were done in 2000 in various Canadian cities comparing methotrexate to mifepristone, after approbation by the federal government. While both drugs had overall similar results, mifepristone was found to act faster.[111] Health Canada gave approval to mifepristone in July 2015.[112] Initially, its use was limited to seven weeks into a pregnancy, but this was changed to nine weeks in 2017. The previous requirement of written consent from the woman was also ended at the same time. It can be dispensed directly to a patient by a pharmacist or a prescribing health professional. Women are required to have an ultrasound to ensure the pregnancy is not ectopic.[113]
Mifepristone was registered for use in Azerbaijan, Georgia, and Uzbekistan in 2002, in Guyana and Moldova in 2004, in Mongolia in 2005, and in Armenia in 2007.[65][114]
Low dose mifepristone tablets (Bi Yun, Fu Nai Er, Hou Ding Nuo, Hua Dian, Si Mi An) for emergency contraception are available directly from a pharmacist without a prescription and with a prescription in China.[21][22][23]
Low dose mifepristone tablets for emergency contraception are available by prescription in Armenia (Gynepriston), Russia (Agesta, Gynepriston, Mifepristone 72, Negele), Ukraine (Gynepriston), and Vietnam (Mifestad 10, Ciel EC).[21][22][23]
Controversy
Many anti-abortion groups in the United States actively campaigned against the approval of mifepristone[115][116][117] and continue to actively campaign for its withdrawal.[118] They cite either ethical issues with abortion or safety concerns regarding the drug and the adverse reactions associated with it.[119] Religious and anti-abortion groups outside the United States have also protested mifepristone, especially in Germany[120] and Australia.[121][122]
Research
The original target for the research group was the discovery and development of compounds with antiglucocorticoid properties.[123] These antiglucocorticoid properties are of great interest in the treatment of severe mood disorders and psychosis, although a review of published articles was inconclusive on their efficacy, and considered the use of these drugs in mood disorders at 'proof of concept' stage.[124]
Use of mifepristone as a cervical ripening agent has been described.[125] The medication has been studied as an antiandrogen in the treatment of prostate cancer.[126][127] Mifepristone showed no detectable anti-HIV activity in clinical trials.[42][45][128][129]
Mifepristone showed initial promise in psychotic major depression, a difficult-to-treat form of depression,[130][131] but a phase-III clinical trial was terminated early due to lack of efficacy.[132] It has been studied in bipolar disorder,[133] post traumatic stress disorder,[131] and anorexia nervosa.[134]
References
- "Mifepristone". The American Society of Health-System Pharmacists. Archived from the original on 22 December 2015. Retrieved 19 December 2015.
- Chen, Melissa J.; Creinin, Mitchell D. (July 2015). "Mifepristone With Buccal Misoprostol for Medical Abortion: A Systematic Review". Obstetrics & Gynecology. 126 (1): 12–21. doi:10.1097/AOG.0000000000000897. ISSN 0029-7844. PMID 26241251.
- Rexrode, edited by Marlene Goldman, Rebecca Troisi, Kathryn (2012). Women and health (2nd ed.). Oxford: Academic. p. 236. ISBN 9780123849793.CS1 maint: extra text: authors list (link)
- Wildschut, H; Both, MI; Medema, S; Thomee, E; Wildhagen, MF; Kapp, N (19 January 2011). "Medical methods for mid-trimester termination of pregnancy". The Cochrane Database of Systematic Reviews (1): CD005216. doi:10.1002/14651858.CD005216.pub2. PMID 21249669.
- "Mifepristone Use During Pregnancy | Drugs.com". Drugs.com. Retrieved 13 March 2018.
- Corey, E.J. (2012). "Mifepristone". Molecules and Medicine. John Wiley & Sons. ISBN 9781118361733. Archived from the original on 8 September 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Kingston, Anne (5 February 2017). "How the arrival of the abortion pill reveals a double standard". Maclean's. Archived from the original on 21 February 2017. Retrieved 21 February 2017.
- Hussein, edited by Julia; McCaw-Binns, Affette; Webber, Roger (2012). Maternal and perinatal health in developing countries. Wallingford, Oxfordshire: CABI. p. 104. ISBN 9781845937461. Archived from the original on 8 September 2017.CS1 maint: extra text: authors list (link)
- Winikoff, B; Sheldon, W (September 2012). "Use of medicines changing the face of abortion". International Perspectives on Sexual and Reproductive Health. 38 (3): 164–6. doi:10.1363/3816412. PMID 23018138.
- Exelgyn (25 March 2015). "Mifegyne Summary of Product Characteristics (SPC)" (PDF). London: Medicines and Healthcare Products Regulatory Agency (MHRA). Archived from the original (PDF) on 2 June 2016. Retrieved 4 April 2016.
- U.S. Food and Drug Administration (30 March 2016). "Mifeprex (mifepristone) Information". Silver Spring, Md.: U.S. Food and Drug Administration. Archived from the original on 3 April 2016. Retrieved 4 April 2016.
- Royal College of Obstetricians and Gynaecologists (23 November 2011). "The Care of Women Requesting Induced Abortion. Evidence-based Clinical Guideline No. 7, 3rd revised edition" (PDF). London: RCOG Press. pp. 68–75. Archived (PDF) from the original on 14 November 2015. Retrieved 4 April 2016.
- World Health Organization (21 June 2012). Safe abortion: technical and policy guidance for health systems, 2nd edition (PDF). Geneva: WHO. pp. 113–116. ISBN 978-92-4-154843-4. Archived (PDF) from the original on 29 March 2016. Retrieved 4 April 2016.
- World Health Organization (10 January 2014). Clinical practice handbook for safe abortion (PDF). Geneva: WHO. ISBN 978-92-4-154871-7. Archived (PDF) from the original on 14 April 2016. Retrieved 4 April 2016.
- American College of Obstetricians and Gynecologists (June 2013). "ACOG Practice Bulletin Number 135: Second-Trimester Abortion" (PDF). Obstetrics & Gynecology. 121 (6): 1394–1406. doi:10.1097/01.AOG.0000431056.79334.cc. PMID 23812485. Archived (PDF) from the original on 14 November 2015. Retrieved 4 April 2016.
- American College of Obstetricians and Gynecologists (March 2014). "ACOG Practice Bulletin Number 143: Medical Management of First-Trimester Abortion" (PDF). Obstetrics & Gynecology. 123 (3): 676–692. doi:10.1097/01.AOG.0000444454.67279.7d. PMID 24553166. Archived (PDF) from the original on 26 June 2016. Retrieved 4 April 2016.
- Grossman, D; White, K; Harris, L; Reeves, M; Blumenthal, PD; Winikoff, B; Grimes, DA (September 2015). "Continuing pregnancy after mifepristone and "reversal" of first-trimester medical abortion: a systematic review". Contraception. 92 (3): 206–11. doi:10.1016/j.contraception.2015.06.001. PMID 26057457.
- Corcept Therapeutics (June 2013). "Korlym prescribing information" (PDF). Menlo Park, Calif.: Corcept Therapeutics. Archived from the original (PDF) on 15 March 2016. Retrieved 4 April 2016.
- Ciato, Denis; Mumbach, Aizhar G.; Paez-Pereda, Marcelo; Stalla, Günter K. (8 December 2016). "Currently used and investigational drugs for Cushing´s disease". Expert Opinion on Investigational Drugs. 26 (1): 75–84. doi:10.1080/13543784.2017.1266338. PMID 27894193.
- Trussell, James; Cleland, Kelly (13 February 2013). "Dedicated emergency contraceptive pills worldwide" (PDF). Princeton: Office of Population Research, Princeton University. Archived (PDF) from the original on 4 March 2016. Retrieved 4 April 2016.
- ICEC (2016). "EC pill types and countries of availability, by brand". New York: International Consortium for Emergency Contraception. Archived from the original on 5 April 2016. Retrieved 4 April 2016.
- Trussell, James; Raymond, Elizabeth G.; Cleland, Kelly (March 2016). "Emergency Contraception: A Last Chance to Prevent Unintended Pregnancylocation=Princeton" (PDF). Office of Population Research, Princeton University. Archived (PDF) from the original on 23 September 2010. Retrieved 7 April 2016.
- Henkel, Andrea; Shaw, Kate A. (December 2018). "Advances in the management of early pregnancy loss". Current Opinion in Obstetrics and Gynecology. 30 (6): 419–424. doi:10.1097/GCO.0000000000000501. PMID 30299321.
- Murji, Ally; Whitaker, Lucy; Chow, Tiffany L; Sobel, Mara L (26 April 2017). Cochrane Gynaecology and Fertility Group (ed.). "Selective progesterone receptor modulators (SPRMs) for uterine fibroids". Cochrane Database of Systematic Reviews. 4: CD010770. doi:10.1002/14651858.CD010770.pub2. PMC 6478099. PMID 28444736.
- Cleland, Kelly (2013). "Significant Adverse Events and Outcomes After Medical Abortion". Obstetrics and Gynecology. 121 (1): 166–171. doi:10.1097/AOG.0b013e3182755763. PMC 3711556. PMID 23262942.
- Friedlander, EmmaKate B.; Soon, Reni; Salcedo, Jennifer; Davis, James; Tschann, Mary; Kaneshiro, Bliss (September 2018). "Prophylactic Pregabalin to Decrease Pain During Medication Abortion: A Randomized Controlled Trial". Obstetrics & Gynecology. 132 (3): 612–618. doi:10.1097/AOG.0000000000002787. ISSN 0029-7844. PMC 6105469. PMID 30095762.
- "Mifeprex label" (PDF). FDA. 19 July 2005. Archived from the original (PDF) on 28 June 2006. Retrieved 22 August 2006.
- Lawton BA, Rose SB, Shepherd J (April 2006). "Atypical presentation of serious pelvic inflammatory disease following mifepristone-induced medical abortion". Contraception. 73 (4): 431–2. doi:10.1016/j.contraception.2005.09.003. PMID 16531180.
- "Mifepristone U.S. Postmarketing Adverse Events Summary through 04/30/2011" (PDF). Archived (PDF) from the original on 18 January 2012. Retrieved 14 November 2011.
- "www.ich.org" (PDF). Archived (PDF) from the original on 28 December 2013.
- Paul, Maureen; Lichtenberg, Steve; Borgatta, Lynn; Grimes, David A.; Stubblefield, Phillip G.; Creinin, Mitchell D. (24 August 2011). Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. John Wiley & Sons. ISBN 9781444358476. Archived from the original on 8 September 2017.
- "CADTH Canadian Drug Expert Committee Final Recommendation Mifepristone and Misoprostol". 18 April 2017. PMID 30512906. Cite journal requires
|journal=(help) - Bhatti, KZ; Nguyen, AT; Stuart, GS (12 November 2017). "Medical Abortion Reversal: Science and Politics Meet". American Journal of Obstetrics and Gynecology. 218 (3): 315.e1–315.e6. doi:10.1016/j.ajog.2017.11.555. PMID 29141197.
- "As controversial 'abortion reversal' laws increase, researcher says new data shows protocol can work". Retrieved 23 April 2018.
- "California Board of Nursing Sanctions Unproven Abortion 'Reversal' (Updated) - Rewire". Rewire. Retrieved 23 November 2017.
- "Controversial 'Abortion Reversal' Regimen Is Put To The Test". NPR.org. Retrieved 19 April 2019.
- "Safety Problems Lead To Early End For Study Of 'Abortion Pill Reversal'". NPR.org. Retrieved 6 December 2019.
- Nuclear Receptors as Drug Targets: Design and Biological Evaluation of Small Molecule Modulators of Nuclear Receptor Action. 2006. pp. 46–. ISBN 978-0-549-70288-7.
- Gallagher P, Young AH (March 2006). "Mifepristone (RU-486) treatment for depression and psychosis: a review of the therapeutic implications". Neuropsychiatr Dis Treat. 2 (1): 33–42. PMC 2671735. PMID 19412444.
- Loose, Davis S.; Stancel, George M. (2006). "Estrogens and Progestins". In Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. pp. 1541–1571. ISBN 978-0-07-142280-2.
- Schimmer, Bernard P.; Parker, Keith L. (2006). "Adrenocorticotropic Hormone; Adrenocortical Steroids and Their Synthetic Analogs; Inhibitors of the Synthesis and Actions of Adrenocortical Hormones". In in Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. pp. 1587–1612. ISBN 978-0-07-142280-2.
- Fiala C, Gemzel-Danielsson K (July 2006). "Review of medical abortion using mifepristone in combination with a prostaglandin analogue". Contraception. 74 (1): 66–86. doi:10.1016/j.contraception.2006.03.018. PMID 16781264.
- Heikinheimo O, Kekkonen R, Lahteenmaki P (2003). "The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action". Contraception. 68 (6): 421–6. doi:10.1016/S0010-7824(03)00077-5. PMID 14698071.
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM (2005). "Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications". Hum Reprod Update. 11 (3): 293–307. doi:10.1093/humupd/dmi002. PMID 15790602.
- Exelgyn Laboratories (February 2006). "Mifegyne UK Summary of Product Characteristics (SPC)". Archived from the original on 28 September 2007. Retrieved 9 March 2007.
- Danco Laboratories (19 July 2005). "Mifeprex U.S. prescribing information" (PDF). Archived from the original (PDF) on 11 December 2005. Retrieved 9 March 2007.
- Baulieu, Étienne-Émile (1985). "RU 486: An antiprogestin steroid with contragestive activity in women". In Baulieu, Étienne-Émile; Segal, Sheldon J (eds.). The antiprogesin steroid RU 486 and human fertility control (Proceedings of a conference on the antiprogestational compound RU 486, held October 23–25, 1984, in Bellagio, Italy). New York: Plenum Press. pp. 1–25. ISBN 978-0-306-42103-7.
Baulieu, Étienne-Émile (1985). "Contragestion by antiprogestin: a new approach to human fertility control". Abortion: medical progress and social implications (Symposium held at the Ciba Foundation, London, 27–29 November 1984). Ciba Foundation Symposium. 115. London: Pitman. pp. 192–210. doi:10.1002/9780470720967.ch15. ISBN 978-0-272-79815-7. PMID 3849413.
Baulieu, Étienne-Émile (1989). "Contragestion with RU 486: a new approach to postovulatory fertility control (from Meet the experts — Antiprogestins, edited by Baulieu, É-É; Proceedings of a meeting held in Rio de Janeiro, Brazil, 27 October 1988)". Acta Obstetricia et Gynecologica Scandinavica Supplement. 149: 5–8. doi:10.1111/j.1600-0412.1989.tb08041.x. ISSN 0300-8835. PMID 2694738.
Greenhouse, Steven (12 February 1989). "A new pill, a fierce battle". The New York Times Magazine. p. SM22. Archived from the original on 1 September 2017.
Palca J (September 1989). "The pill of choice?". Science. 245 (4924): 1319–23. Bibcode:1989Sci...245.1319P. doi:10.1126/science.2781280. JSTOR 1704254. PMID 2781280.
Baulieu EE (September 1989). "Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor". Science. 245 (4924): 1351–7. Bibcode:1989Sci...245.1351B. doi:10.1126/science.2781282. JSTOR 1704267. PMID 2781282.
Baulieu EE (October 1989). "The Albert Lasker Medical Awards. RU-486 as an antiprogesterone steroid. From receptor to contragestion and beyond". JAMA. 262 (13): 1808–1814. doi:10.1001/jama.262.13.1808. PMID 2674487. Archived from the original on 21 May 2013.
Bonner, Staci (July 1991). "Drug of choice". SPIN. 7 (4): 55–56, 88. ISSN 0886-3032. Archived from the original on 12 May 2016.
Baulieu, Étienne-Émile; Rosenblum, Mort (15 November 1991). The "abortion pill": RU-486: a woman's choice (translation of: 'Génération pilule). New York: Simon & Schuster. pp. 18, 26–28. ISBN 978-0-671-73816-7. Archived from the original on 27 May 2016.
Beck, Joan (2 January 1992). "RU-486 pill adds a new dimension to the abortion debate". Chicago Tribune. p. 25. Archived from the original on 18 February 2013.
Chesler, Ellen (31 July 1992). "RU-486: we need prudence, not politics". The New York Times. p. A27. Archived from the original on 4 March 2016.
Baulieu, Étienne-Émile (13 April 1993). "1993: RU 486—a decade on today and tomorrow". In Donaldson, Molla S.; Dorflinger, Laneta; Brown, Sarah S.; Benet, Leslie Z. (eds.). Clinical applications of mifepristone (RU 486) and other antiprogestins; assessing the science and recommending a research agenda; (Committee on Anti-progestins: Assessing the Science; Division of Health Promotion and Disease Prevention; Institute of Medicine). Washington, D.C.: National Academy Press. pp. 71–119. doi:10.17226/2203. ISBN 978-0-309-04949-8. PMID 25144090. Archived from the original on 27 March 2013.
Baulieu EE (June 1994). "RU486: a compound that gets itself talked about". Hum. Reprod. 9 Suppl 1: 1–6. doi:10.1093/humrep/9.suppl_1.1. PMID 7962455.
Baulieu, Étienne-Émile (1997). "Innovative procedures in family planning". In Johannisson, Elisabeth; Kovács, László; Resch, Bela A; Bruyniks, Nico P (eds.). Assessment of research and service needs in reproductive health in Eastern Europe — concerns and commitments. Proceedings of a workshop organized by the ICRR and the WHO Collaborating Centre on Research in Human Reproduction in Szeged, Hungary, 25–27 October 1993. New York: Parthenon Publishing. pp. 51–60. ISBN 978-1-85070-696-0. Archived from the original on 3 May 2016.
. (2008). "contragestive". The American Heritage medical dictionary. Boston: Houghton Mifflin. p. 124. ISBN 978-0-618-94725-6.CS1 maint: numeric names: authors list (link)adj. Capable of preventing gestation, either by preventing implantation or by causing the uterine lining to shed after implantation. —n. A contragestive drug or agent.
Ammer, Christine (2009). "contragestive". The encyclopedia of women's health (6th ed.). New York: Facts On File. pp. 124–125. ISBN 978-0-8160-7407-5.Also contragestant, abortion pill. A substance called mifepristone, or RU-486, which was developed by Dr. Etienne Baulieu and the Roussel-Uclaf company. The contragestive blocks progesterone receptors in the endometrium (uterine lining), preventing its buildup by progesterone; hence the uterus cannot sustain a pregnancy. It does not prevent fertilization or implantation, so technically it is an ABORTIFACIENT rather than a contraceptive.
- Heikinheimo O (July 1997). "Clinical pharmacokinetics of mifepristone". Clin Pharmacokinet. 33 (1): 7–17. doi:10.2165/00003088-199733010-00002. PMID 9250420.
- Heikinheimo O, Kekkonen R, Lähteenmäki P (December 2003). "The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action". Contraception. 68 (6): 421–6. doi:10.1016/S0010-7824(03)00077-5. PMID 14698071.
- Wang J, Chen J, Wan L, Shao J, Lu Y, Zhu Y, Ou M, Yu S, Chen H, Jia L (March 2014). "Synthesis, spectral characterization, and in vitro cellular activities of metapristone, a potential cancer metastatic chemopreventive agent derived from mifepristone (RU486)". AAPS J. 16 (2): 289–98. doi:10.1208/s12248-013-9559-2. PMC 3933578. PMID 24442753.
- Baulieu, Étienne-Émile; Rosenblum, Mort (1991). The "abortion pill": RU-486, a woman's choice. New York: Simon & Schuster. ISBN 978-0-671-73816-7.
Lader, Lawrence (1991). RU 486: the pill that could end the abortion wars and why American women don't have it. Reading: Addison-Wesley. ISBN 978-0-201-57069-4.
Villaran, Gilda (1998). "RU 486". In Schlegelmilch, Bodo B. (ed.). Marketing ethics: an international perspective. London: Thomson Learning. pp. 155–190. ISBN 978-1-86152-191-0.
Ulmann, André (2000). "The development of mifepristone: a pharmaceutical drama in three acts". J Am Med Womens Assoc. 55 (3 Suppl): 117–20. PMID 10846319. - Teutsch, Georges (24 November 1989). "RU 486 development". Science. 246 (4933): 985. doi:10.1126/science.2587990. PMID 2587990.
Cherfas, J (24 November 1989). "Dispute surfaces over paternity of RU 486". Science. 246 (4933): 994. Bibcode:1989Sci...246..994C. doi:10.1126/science.2587988. PMID 2587988.
Philibert, D; Teutsch, G (9 February 1990). "RU 486 development". Science. 247 (4943): 622. Bibcode:1990Sci...247..622P. doi:10.1126/science.2300819. PMID 2300819.
Ulmann, A; Teutsch, G; Philibert, D (June 1990). "RU 486". Scientific American. Vol. 262 no. 6. pp. 42–8. doi:10.1038/scientificamerican0690-42. PMID 2343294.
Teutsch, G.; Deraedt, R.; Philibert, D. (1993). "Mifepristone". In Lednicer, Daniel (ed.). Chronicles of drug discovery, Vol. 3. Washington, DC: American Chemical Society. pp. 1–43. ISBN 978-0-8412-2523-7.
Teutsch, G; Philibert, D (June 1994). "History and perspectives of antiprogestins from the chemist's point of view". Human Reproduction. 9 (Suppl 1): 12–31. doi:10.1093/humrep/9.suppl_1.12. PMID 7962457.
Sittig, Marshall, ed. (2007). "Mifepristone". Pharmaceutical manufacturing encyclopedia (3rd ed.). Norwich, NY: William Andrew Publishing. pp. 2307–2310. ISBN 978-1-60119-339-1.
US patent 4,386,085, Teutsch, Jean G.; Costerousse, Germain; Philibert, Daniel; Deraedt, Roger, "Novel steroids", issued 1983-05-31 assigned to Roussel Uclaf - Eder, Richard (20 April 1982). "Birth control: 4-day pill is promising in early test". The New York Times. p. C1. Archived from the original on 25 June 2016.
Herrmann, Walter; Wyss, Rolf; Riondel, Anne; Philibert, Daniel; Teutsch, Georges; Sakiz, Edouard; Baulieu, Étienne-Émile (17 May 1982). "The effects of an antiprogesterone steroid in women: interruption of the menstrual cycle and of early pregnancy". Comptes Rendus de l'Académie des Sciences, Série III. 294 (18): 933–8. PMID 6814714. - Kolata, Gina (24 September 1988). "France and China allow sale of a drug for early abortion". The New York Times. p. A1. Archived from the original on 1 September 2017.
- Greenhouse, Steven (27 October 1988). "Drug maker stops all distribution of abortion pill". The New York Times. p. A1. Archived from the original on 5 March 2016.
- Greenhouse, Steven (29 October 1988). "France ordering company to sell its abortion drug". The New York Times. p. A1. Archived from the original on 4 March 2016.
- Smith, W. (September 1991). "Great Britain second country to allow use of RU-486". Plan Parent Eur. 20 (2): 20. PMID 12284548.
- . (December 1992). "RU 486 licensed in Sweden". IPPF Med Bull. 26 (6): 6. PMID 12346922.CS1 maint: numeric names: authors list (link)
- Newman, Barry (22 February 1993). "Drug dilemma: among those wary of abortion pill is maker's parent firm; Germany's Hoechst is facing pressure from Clinton to sell RU-486 in U.S.". The Wall Street Journal. p. A1.
"F.D.A. says company delays abortion pill". The New York Times. Associated Press. 16 April 1993. p. A14. Archived from the original on 3 August 2016.
Jouzaitis, Carol (17 October 1994). "Abortion pill battle surprises French firm". Chicago Tribune. p. 1 (Business). Archived from the original on 18 February 2013. - Seelye, Katharine Q. (17 May 1994). "Accord opens way for abortion pill in U.S. in 2 years". The New York Times. p. A1. Archived from the original on 3 August 2016.
- Kolata, Gina (29 September 2000). "U.S. approves abortion pill; drug offers more privacy and could reshape debate". The New York Times. p. A1. Archived from the original on 4 August 2017.
- Moore, Stephen D.; Kamm, Thomas; Fleming, Charles (11 December 1996). "Hoechst to seek rest of Roussel-Uclaf; expected $3.04 billion offer would add to the wave of drug-sector linkups". The Wall Street Journal. p. A3.
Marshall, Matt (11 December 1996). "Hoechst offers to pay $3.6 billion for rest of Roussel". The Wall Street Journal. p. A8.
Bloomberg Business News (11 December 1996). "Hoechst to buy rest of Roussel". The New York Times. p. D4. Archived from the original on 25 June 2016. - Bloomberg News (9 April 1997). "Pill for abortion ends production". The New York Times. p. D2. Archived from the original on 25 June 2016.
Jouzaitis, Carol (9 April 1997). "Abortion pill maker bows to boycott heat; German firm gives up RU-486 patent; little impact likely in U.S." Chicago Tribune. p. 4. Archived from the original on 18 February 2013.
Lavin, Douglas (9 April 1997). "Hoechst will stop making abortion pill". The Wall Street Journal. p. A3.
. (18 April 1997). "Roussel-Uclaf to transfer RU 486 rights". Reprod Freedom News. 6 (7): 8. PMID 12292550.CS1 maint: numeric names: authors list (link)
Dorozynski, Alexander (19 April 1997). "Boycott threat forces French company to abandon RU486". BMJ. 314 (7088): 1150. doi:10.1136/bmj.314.7088.1145m. PMC 2126515. PMID 9146386. - . (November 4, 2009). "List of mifepristone approval" (PDF). New York: Gynuity Health Projects. Archived from the original (PDF) on July 26, 2011. Retrieved May 4, 2018.CS1 maint: numeric names: authors list (link)
. (November 4, 2009). "Map of mifepristone approval" (PDF). New York: Gynuity Health Projects. Archived from the original (PDF) on July 26, 2011. Retrieved June 11, 2010.CS1 maint: numeric names: authors list (link) - excluding Alabama, California, Connecticut, Washington, D.C., Florida, Georgia, Hawaii, Illinois, Kentucky, Louisiana, Massachusetts, Maryland, Nebraska, Nevada, New Hampshire, Rhode Island, Tennessee, and Wisconsin
- Pazol, Karen; Zane, Suzanne B.; Parker, Wilda, Y.; Hall, Laura R.; Gamble, Sonya B.; Hamdan, Saeed; Berg, Cynthia; Cook, Douglas A.; Division of Reproductive Health (25 February 2011). "Abortion surveillance — United States, 2007" (PDF). MMWR Surveill Summ. 60 (1): 1–44. PMID 21346710. Archived (PDF) from the original on 6 April 2011.CS1 maint: multiple names: authors list (link)
- Jones, Rachel K.; Kooistra, Kathryn (March 2011). "Abortion incidence and access to services in the United States, 2008" (PDF). Perspect Sex Reprod Health. 43 (1): 41–50. doi:10.1363/4304111. PMID 21388504. Archived (PDF) from the original on 27 September 2011.
Stein, Rob (11 January 2011). "Decline in U.S. abortion rate stalls". The Washington Post. p. A3. Archived from the original on 4 March 2016. - Fjerstad, Mary; Trussell, James; Sivin, Irving; Lichtenberg, E. Steve; Cullins, Vanessa (9 July 2009). "Rates of serious infection after changes in regimens for medical abortion". New England Journal of Medicine. 361 (2): 145–151. doi:10.1056/NEJMoa0809146. PMC 3568698. PMID 19587339.
Allday, Erin (9 July 2009). "Change cuts infections linked to abortion pill". San Francisco Chronicle. p. A1. - Jones, Rachel K.; Jerman, Jenna (March 2017). "Abortion Incidence and Service Availability In the United States 2014". Perspectives on Sexual and Reproductive Health. 49–1 (1): 17–27. doi:10.1363/psrh.12015. PMC 5487028. PMID 28094905.
- Vilain, Annick (December 2009). "Voluntary terminations of pregnancies in 2007" (PDF). DREES, Ministry of Health. Archived from the original (PDF) on 31 March 2010. Retrieved 9 June 2010.
- Department of Health (25 May 2010). "Abortion statistics, England and Wales: 2009". Department of Health (United Kingdom). Archived from the original on 15 November 2010. Retrieved 9 June 2010.
- ISD Scotland (25 May 2010). "Abortion Statistics, year ending December 2009". Information Services Division (ISD), NHS National Services Scotland. Retrieved 9 June 2010.
- National Board of Health and Welfare, Sweden (12 May 2010). "Induced Abortions 2010" (PDF). National Board of Health and Welfare, Sweden. Archived from the original (PDF) on 27 July 2011. Retrieved 9 June 2010.
- "FDA Approves Mifepristone for the Termination of Early Pregnancy". FDA press release/U.S. Gov. 2000. Archived from the original on 10 September 2006. Retrieved 27 April 2009.
- "The abortion pill Mifegyne tested for adverse reactions". Danish Medicines Agency. 27 July 2005. Retrieved 20 September 2006.
- "FDA approval letter for Mifepristone". FDA. 28 September 2000. Archived from the original on 16 November 2001. Retrieved 16 September 2006.
- "Medication Abortion in the United States: Mifepristone Fact Sheet" (PDF). Gynuity Health Projects. 2005. Archived from the original (PDF) on 24 September 2007.
- Klitsch M (November–December 1991). "Antiprogestins and the abortion controversy: a progress report". Fam Plann Perspect. 23 (6): 275–82. doi:10.2307/2135779. JSTOR 2135779. PMID 1786809.
- Nancy Gibbs (2 October 2000). "The Pill Arrives". Cnn.com. Archived from the original on 6 October 2006. Retrieved 20 September 2006.
- Tamar Lewin (30 January 1995). "Clinical Trials Giving Glimpse of Abortion Pill". The New York Times. Archived from the original on 28 November 2007. Retrieved 20 September 2006.
- Tamar Lewin (13 November 1997). "Lawsuits' Settlement Brings New Hope for Abortion Pill". The New York Times. Archived from the original on 10 February 2007. Retrieved 16 September 2006.
- Sharon Lerner (August 2000). "RU Pissed Off Yet?". The Village Voice. Archived from the original on 7 November 2006. Retrieved 16 September 2006.
- Danco Laboratories (29 March 2016). "Mifeprex prescribing information" (PDF). Silver Spring, Md.: U.S. Food and Drug Administration. Archived (PDF) from the original on 30 March 2016.
- American Congress of Obstetricians and Gynecologists (30 March 2016). "ACOG Statement on Medication Abortion". Washington, D.C.: ACOG. Archived from the original on 3 April 2016. Retrieved 7 April 2016.
- "Postmarket Drug Safety Information for Patients and Providers". US Food & Drug Administration. 30 March 2016.
- North, Anna (20 August 2019). "The first generic abortion pill just hit the US market. Here's what that means". Vox. Retrieved 14 July 2020.
- Woodcock, Janet (12 May 2006). "Testimony on RU-486". Committee on Government Reform, House of Representatives. FDA. Archived from the original on 27 September 2006. Retrieved 19 August 2006.
- Christin-Maitre, S., Bouchard, P., Spitz, I. M. (2000). "Medical termination of pregnancy". New England Journal of Medicine. 342 (13): 946–56. doi:10.1056/NEJM200003303421307. PMID 10738054.CS1 maint: multiple names: authors list (link)
- Stojnic J, et al. (2006). "Medicamentous abortion with mifepristone and misoprostol in Serbia and Montenegro". Vojnosanitetski Pregled. Military-medical and Pharmaceutical Review. 63 (6): 558–63. doi:10.2298/VSP0606558S. PMID 16796021.
- "Archived copy". Archived from the original on 26 September 2017. Retrieved 28 September 2017.CS1 maint: archived copy as title (link)
- "Abortion pill approved in Italy". BBC News. 31 July 2009. Retrieved 31 July 2009.
- "Abortion pill sparks bitter protest". The Budapest Times. 19 September 2005. Archived from the original on 11 January 2007. Retrieved 16 September 2006.
- Peter S. Green (24 June 2003). "A Rocky Landfall for a Dutch Abortion Boat". The New York Times. Archived from the original on 10 February 2007. Retrieved 16 September 2006.
- Linepharma (7 November 2014). "Mifepristone Linepharma Summary of Product Characteristics (SPC)" (PDF). London: Medicines and Healthcare Products Regulatory Agency (MHRA). Archived from the original (PDF) on 2 June 2016. Retrieved 14 April 2016.
- Sun Pharmaceuticals (4 March 2015). "Medabon Summary of Product Characteristics (SPC)" (PDF). London: Medicines and Healthcare Products Regulatory Agency (MHRA). Archived from the original (PDF) on 3 June 2016. Retrieved 4 April 2016.
- "Marie Stopes International Australia – Medical Abortion". 2010. Archived from the original on 22 November 2010. Retrieved 15 December 2010.
- "Abortion pill – RU486 (mifepristone)". Better Health Channel Victoria. July 2010. Archived from the original on 14 August 2010. Retrieved 15 December 2010.
- MS Health (24 December 2014). "Mifepristone Linepharma (MS-2 Step) 200 mg tablet product information". Symonston, Australian Capital Territory, Australia: Therapeutic Goods Administration. Archived from the original on 8 September 2017. Retrieved 4 April 2016.
- MS Health (12 May 2015). "Mifepristone Linepharma 200 mg tablet product information". Symonston, Australian Capital Territory, Australia: Therapeutic Goods Administration. Retrieved 4 April 2016.
- Sparrow MJ (2004). "A woman's choice". Aust NZ J Obstet Gynaecol. 44 (2): 88–92. doi:10.1111/j.1479-828X.2004.00190.x. PMID 15089829.
- Etienne-Emile Baulieu; Daniel S. Seidman; Selma Hajri (October 2001). "Mifepristone(RU-486) and voluntary termination of pregnancy: enigmatic variations or anecdotal religion-based attitudes?". Human Reproduction. 16 (10): 2243–2244. doi:10.1093/humrep/16.10.2243. PMID 11574524. Archived from the original on 21 March 2006. Retrieved 16 September 2006.
- Ulmann A (2000). "The development of mifepristone: a pharmaceutical drama in three acts". J Am Med Women's Assoc. 55 (3 Suppl): 117–20. PMID 10846319.
- Wu S (2000). "Medical abortion in China". J Am Med Women's Assoc. 55 (3 Suppl): 197–9, 204. PMID 10846339.
- "Family planning in China: RU-486, abortion, and population trends". U.S. Embassy Beijing. 2000. Archived from the original on 11 March 2002. Retrieved 14 September 2006.
- Tsai EM, Yang CH, Lee JN (2002). "Medical abortion with mifepristone and misoprostol: a clinical trial in Taiwanese women". J Formos Med Assoc. 101 (4): 277–82. PMID 12101864.
- Ganatra B, Bygdeman M, Nguyen DV, Vu ML, Phan BT (2004). "From research to reality: the challenges of introducing medical abortion into service delivery in Vietnam". Reprod Health Matters. 12 (24): 105–13. doi:10.1016/S0968-8080(04)24022-8. PMID 15938163.
- "Medical Abortion-Implications for Africa". Ipas. 2003. Archived from the original on 28 September 2007. Retrieved 16 September 2006.
- Hajri S (2004). "Medication abortion: the Tunisian experience". Afr J Reprod Health. 8 (1): 63–9. doi:10.2307/3583307. hdl:1807/3883. JSTOR 3583307. PMID 15487615.
- "Mifepristone can be sold only to approved MTP Centres: Rajasthan State HRC". Indian Express Health Care Management. 2000. Archived from the original on 24 January 2012.
- ."Results of the Canadian trials of RU486, the 'Abortion Pill'." (n.d.). Retrieved 2006-12-08.
- "RU-486 abortion pill approved by Health Canada". Archived from the original on 31 July 2015. Retrieved 30 July 2015.
- "Health Canada eases restrictions on abortion pill Mifegymiso | CBC News". CBC. Retrieved 28 April 2018.
- "Medication Abortion". Ibis. 2002. Archived from the original on 4 November 2006. Retrieved 19 September 2006.
- Paige Comstock Cunningham; Leanne McCoy; Clarke D. Ferguson (28 February 1995). "Citizen Petition to the U.S. Food and Drug Administration". Americans United for Life. Archived from the original on 3 October 2006. Retrieved 20 September 2006.
- Margaret Talbot (11 July 1999). "The Little White Bombshell". The New York Times. Retrieved 20 September 2006.
- "Abortion Foes to Boycott Drugs (Altace) Made By RU-486 Manufacturer". The Virginia Pilot. 8 July 1994. Archived from the original on 20 February 2008. Retrieved 15 September 2006.
- Stan Guthrie (11 June 2001). "Counteroffensive Launched on RU-486". Christianity Today. Archived from the original on 20 September 2006. Retrieved 20 September 2006.
- Gina Kolata (24 September 2003). "Death at 18 Spurs Debate Over a Pill For Abortion". The New York Times. Retrieved 20 September 2006.
- John L. Allen (12 February 1999). "Abortion debates rock Germany: introduction of abortion pill exacerbates controversy". National Catholic Reporter. Archived from the original on 28 May 2005. Retrieved 14 September 2006.
- "Catholic and Evangelical students join Muslims in RU-486 fight". Catholic News. 9 February 2006. Archived from the original on 27 October 2006. Retrieved 18 September 2006.
- "Death Toll Rises to 11 Women". Australians Against RU-486. 2006. Archived from the original on 20 August 2006. Retrieved 20 September 2006.
- Hazra BG, Pore VS (2001). "Mifepristone (RU-486), the recently developed antiprogesterone drug and its analogues". J Indian Inst Sci. 81: 287–98.
- Peter Gallagher; Navdeep Malik; James Newham; Allan H Young; Nicol Ferrier; Paul Mackin (2008). MacKin, Paul (ed.). "Antiglucocorticoid treatments for mood disorders". Cochrane Database of Systematic Reviews (1): CD005168. doi:10.1002/14651858.CD005168.pub2. PMID 18254070. (Retracted, see doi:10.1002/14651858.cd005168.pub3. If this is an intentional citation to a retracted paper, please replace
{{Retracted}}with{{Retracted|intentional=yes}}.) - Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK (May 2006). "Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events". Am. J. Obstet. Gynecol. 194 (5): 1391–8. doi:10.1016/j.ajog.2005.11.026. PMID 16647925.
- Taplin ME, Manola J, Oh WK, Kantoff PW, Bubley GJ, Smith M, Barb D, Mantzoros C, Gelmann EP, Balk SP (2008). "A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones". BJU Int. 101 (9): 1084–9. doi:10.1111/j.1464-410X.2008.07509.x. PMID 18399827.
- "Mifepristone - Corcept Therapeutics - AdisInsight".
- Flexner C (December 2007). "HIV drug development: the next 25 years". Nat Rev Drug Discov. 6 (12): 959–66. doi:10.1038/nrd2336. PMID 17932493.
- Tang OS, Ho PC (2006). "Clinical applications of mifepristone". Gynecol Endocrinol. 22 (12): 655–9. doi:10.1080/09513590601005946. PMID 17162706.
- Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF (October 2001). "Rapid reversal of psychotic depression using mifepristone". J Clin Psychopharmacol. 21 (5): 516–21. doi:10.1097/00004714-200110000-00009. PMID 11593077.
- Howland, Robert H. (June 2013). "Mifepristone as a therapeutic agent in psychiatry". Journal of Psychosocial Nursing and Mental Health Services. 51 (6): 11–14. doi:10.3928/02793695-20130513-01. ISSN 0279-3695. PMID 23814820.
- Damian Gard for Fierce Biotech. May 7, 2014. Corcept tanks as depression drug comes up short in Phase III Archived March 2, 2016, at the Wayback Machine
- Soria, Virginia; González-Rodríguez, Alexandre; Huerta-Ramos, Elena; Usall, Judith; Cobo, Jesús; Bioque, Miquel; Barbero, Juan David; García-Rizo, Clemente; Tost, Meritxell (July 2018). "Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: a systematic review and narrative synthesis". Psychoneuroendocrinology. 93: 8–19. doi:10.1016/j.psyneuen.2018.04.012. PMID 29680774.
- Bou Khalil, Rami; Souaiby, Lama; Farès, Nassim (March 2017). "The importance of the hypothalamo-pituitary-adrenal axis as a therapeutic target in anorexia nervosa". Physiology & Behavior. 171: 13–20. doi:10.1016/j.physbeh.2016.12.035. PMID 28043861.