Exaprolol
 | |
| Names | |
|---|---|
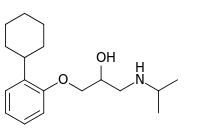
| IUPAC name
1-(2-Cyclohexylphenoxy)-3-(propan-2-ylamino)propan-2-ol | |
| Other names
Esprolol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C18H29NO2 | |
| Molar mass | 291.435 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Exaprolol is a beta-adrenoceptor antagonist.[1]
Synthesis
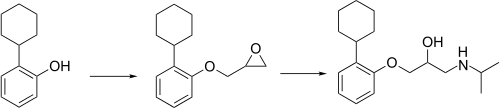
Exaprolol synthesis[2]
gollark: Or at least not secure in some contexts; it's fine for the potatOS use as far as I can tell.
gollark: It's probably not very secure as it doesn't use a standard curve or whatever and has no side channel attack mitigations, but OH WELL.
gollark: PotatOS uses some ECC libraries for verification of things.
gollark: > Could you provide working cryptographic signing api that would work in CC lua?That actually does exist.
gollark: Okay!!!!!?!?¡!!!¡¿!!1!1
References
- Van Waarde, A; Doorduin, J; De Jong, JR; Dierckx, RA; Elsinga, PH (2008). "Synthesis and preliminary evaluation of (S)-11C-exaprolol, a novel beta-adrenoceptor ligand for PET". Neurochemistry International. 52 (4–5): 729–33. doi:10.1016/j.neuint.2007.09.009. PMID 17961850.
- Carissimi, M; Gentili, P; Grumelli, E; Milla, E; Picciola, G; Ravenna, F (1976). "Basic ethers of cyclohexylphenols with beta-blocking activity: Synthesis and pharmacological study of exaprolol". Arzneimittel-Forschung. 26 (4): 506–16. PMID 8056.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.