Exaprolol
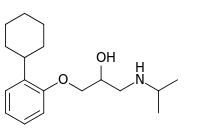 | |
| Names | |
|---|---|
| IUPAC name
1-(2-Cyclohexylphenoxy)-3-(propan-2-ylamino)propan-2-ol | |
| Other names
Esprolol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C18H29NO2 | |
| Molar mass | 291.435 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Exaprolol is a beta-adrenoceptor antagonist.[1]
Synthesis
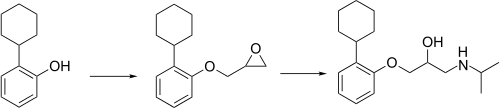
Exaprolol synthesis[2]
gollark: ```You try to write the name, but several characters disappear as you write, so you decide to try something else.The name you tried to use contains invalid characters. Names can only contain letters, numbers, spaces, apostrophes, and dashes.-XTypedHoles```What?!
gollark: I would automate it, but TJ09.
gollark: I forgot to get lots of shards this week. Instead of actual playing, I can just click a few buttons mindlessly and get my shard cap filled.
gollark: By "massbreed" I mean "breed my ~10"; not very "mass".
gollark: Also, two shards per breeding? Seems poorly thought out, since you can just earn a stupid amount by massbreeding to AP.
References
- Van Waarde, A; Doorduin, J; De Jong, JR; Dierckx, RA; Elsinga, PH (2008). "Synthesis and preliminary evaluation of (S)-11C-exaprolol, a novel beta-adrenoceptor ligand for PET". Neurochemistry International. 52 (4–5): 729–33. doi:10.1016/j.neuint.2007.09.009. PMID 17961850.
- Carissimi, M; Gentili, P; Grumelli, E; Milla, E; Picciola, G; Ravenna, F (1976). "Basic ethers of cyclohexylphenols with beta-blocking activity: Synthesis and pharmacological study of exaprolol". Arzneimittel-Forschung. 26 (4): 506–16. PMID 8056.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.