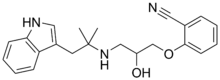
Bucindolol
Bucindolol is a non-selective beta blocker with additional weak alpha-blocking properties and some intrinsic sympathomimetic activity.[1][2] It was under review by the FDA in the United States for the treatment of heart failure in 2009, but was rejected due to issues pertaining to integrity of data submitted.[1][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.114.291 |
| Chemical and physical data | |
| Formula | C22H25N3O2 |
| Molar mass | 363.461 g·mol−1 |
| 3D model (JSmol) | |
| |
See also
References
- "formularyjournal.modernmedicine.com". Archived from the original on 2012-03-04.
- Willette RN, Mitchell MP, Ohlstein EH, Lukas MA, Ruffolo RR (January 1998). "Evaluation of intrinsic sympathomimetic activity of bucindolol and carvedilol in rat heart". Pharmacology. 56 (1): 30–6. doi:10.1159/000028179. PMID 9467185.
- "FDA rejects bucindolol and questions trial integrity « CardioBrief".
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.