Chloroprocaine
Chloroprocaine (trade name Nesacaine, Nesacaine-MPF) (often in the hydrochloride salt form as the aforementioned trade names) is a local anesthetic given by injection during surgical procedures and labor and delivery. Chloroprocaine vasodilates; this is in contrast to cocaine which vasoconstricts. Chloroprocaine is an ester anesthetic.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
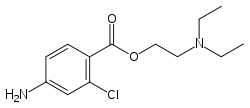
| Formula | C13H19ClN2O2 |
| Molar mass | 270.76 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Uses
Chloroprocaine is used for regional anaesthesia including spinal anaesthesia, caudal anaesthesia and epidural anesthesia[2][3]
It is also indicated for local anaesthesia including brachial plexus block, cervical nerve block, occipital nerve block. mandibular nerve block or maxillary nerve block for dental anesthesia, ophthalmic anesthesia via infraorbital nerve block, ulnar nerve block, paravertebral block, intercostal nerve block, sciatic nerve block, stellate ganglion block, lumbar sympathetic block and interdigital block.[3]
It is also used for obstetric anesthesia including pudendal nerve block and paracervical block.[3]
Subarachnoid block
Chloroprocaine was developed to meet the need for a short-acting spinal anaesthetic that is reliable and has a favourable safety profile to support the growing need for day-case surgery. Licensed in Europe for surgical procedures up to 40 minutes, chloroprocaine is an ester-type local anaesthetic with the shortest duration of action of all the established local anaesthetics. It has a significantly shorter duration of action than lidocaine and is significantly less toxic. Chloroprocaine has a motor block lasting for 40 minutes, a rapid onset time of 3–5 minutes (9.6 min ± 7.3 min at 40 mg dose; 7.9 min ± 6.0 min at 50 mg dose) and a time to ambulation of 90 minutes without complications, especially lacking transient neurologic symptomatology.
These data are based upon a retrospective review of 672 patients suitable for spinal anaesthesia in surgical procedures less than 60 minutes' duration using 30–40 mg chloroprocaine. The results showed good surgical anaesthesia, a fast onset time, and postoperative mobilization after 90 minutes without complications.[4]
The use of chloroprocaine in the subarachnoid space has been questioned.[5] In the early 1980s, several cases were reported of neurological deficits after inadvertent intrathecal injections intended for epidural delivery.[6] These doses were an order of magnitude higher than is currently used for intrathecal delivery.[7][8][9] It is also thought that these deficits were also related to the preservative sodium bisulfate,[10][11] although this is also controversial.[12][13]
In recent years, several studies have been published on the safe use of intrathecal chloroprocaine when appropriate dosage is used and with preservative-free preparations.[14][15]
It is currently approved for intrathecal use in the United States [16] and in Europe.[17]
Obstetrics
Amide-linked local anesthetic agents, such as lidocaine and bupivacaine, can become "trapped" in their ionized forms on the fetal side of the placenta, so their net transfer across the placenta is increased. An ester-linked local anesthetic agent, such as 2-chloroprocaine, is rapidly metabolized, and placental transfer is limited. Since the metabolism of 2-chloroprocaine by fetal plasma is slower than in maternal plasma, the potential for ion trapping exists. Fetal pH is slightly lower than maternal (7.32 to 7.38), thus most unionized drugs are "ion trapped" to a degree, even in a healthy fetus. Chloroprocaine (pKa 8.7) is the drug of choice for epidural analgesia and a decompensating fetus, because it does not participate in ion trapping. Placental transfer of 2-chloroprocaine is not influenced by fetal acidosis.[18]
The in vitro half-life of chloroprocaine is 21 seconds for maternal and 43 seconds for fetal blood. In patients who are homozygous atypical for plasma cholinesterase, chloroprocaine typically exists for two minutes in circulation.[19][20]
It is not used in intravenous regional anesthesia due to the risk of thrombophlebitis.
Synthesis

The hydrochloride salt of 4-amino-2-chlorobenzoyl chloride is made by the reaction of 2-chloro-4-aminobenzoic acid with thionyl chloride. Synthesis of this drug is then accomplished by directly reacting the product of the last step with the hydrochloride salt of 2-diethylaminoethanol.
See also
References
- Drug bank entry for Chloroprocaine
- Sintetica Limited (9 March 2017). "Ampres 10 mg/ml solution for injection". EMC.
- Physicians' Desk Reference. "chloroprocaine hydrochloride". USA: PDR.net.
- Ampres (chloroprocaine) Summary of Product Characteristics, Palas T, Cloroprocaina in chirurgia ambulatoriale: uno studio osservazionale, Perimed 2009, 3(2):31-34
- Drasner K. Chloroprocaine spinal anesthesia: back to the future? Anes analg 2005; 100: 549-52.
- Reisner, Laurence S., Bruce N. Hochman, and Michael H. Plumer. "Persistent neurologic deficit and adhesive arachnoiditis following intrathecal 2-chloroprocaine injection." Anesthesia & Analgesia 59.6 (1980): 452-454
- Förster, J. G., et al. "Chloroprocaine vs. articaine as spinal anaesthetics for day‐case knee arthroscopy." Acta Anaesthesiologica Scandinavica 55.3 (2011): 273-281.
- Förster, J. G., et al. "Chloroprocaine 40 mg produces shorter spinal block than articaine 40 mg in day‐case knee arthroscopy patients." Acta Anaesthesiologica Scandinavica (2013).
- Lacasse, Marie-Andrée, et al. "Comparison of bupivacaine and 2-chloroprocaine for spinal anesthesia for outpatient surgery: a double-blind randomized trial." Canadian Journal of Anesthesia/Journal canadien d'anesthésie 58.4 (2011): 384-391.
- Gissen, Aaron J., Sanjay Datta, and Donald Lambert. "The Chloroprocaine Controversy: II. Is Chloroprocaine Neurotoxic?." Regional Anesthesia and Pain Medicine 9.3 (1984): 135-145.
- Wang, B. C., et al. "Lumbar Subarachnoid Ethylenediaminetetraacetate Induces Hindlimb Tetanic Contractions in Rats Prevention by CaCl2 Pretreatment; Observation of Spinal Nerve Root Degeneration." Anesthesia & Analgesia 75.6 (1992): 895-899.
- Taniguchi, Masahiko, Andrew W. Bollen, and Kenneth Drasner. "Sodium bisulfite: scapegoat for chloroprocaine neurotoxicity?." Anesthesiology 100.1 (2004): 85-91.
- Cabré, Francesc, et al. "Occurrence and comparison of sulfite oxidase activity in mammalian tissues." Biochemical medicine and metabolic biology 43.2 (1990): 159-162.
- Goldblum, E., and A. Atchabahian. "The use of 2‐chloroprocaine for spinal anaesthesia." Acta Anaesthesiologica Scandinavica (2013).
- Förster, J. G., et al. "Chloroprocaine 40 mg produces shorter spinal block than articaine 40 mg in day‐case knee arthroscopy patients." Acta Anaesthesiologica Scandinavica (2013).
- https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=208791
- http://www.anesthesiologynews.com/ViewArticle.aspx?d=Clinical+Anesthesiology&d_id=1&i=June+2013&i_id=962&a_id=23319
- Philipson EH, Kuhnert BR, Syracuse CD. Fetal acidosis, 2-chloroprocaine, and epidural anesthesia for cesarean section. Am J Obstet Gynecol. 1985 Feb 1;151(3):322-4.
- Chestnut: Obstetric Anesthesia, 3rd ed, p333.
- Hughes: Anesthesia for Obstetrics, 4th ed, p75.