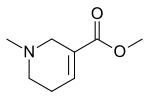
Arecoline
Arecoline (/əˈrɛkəliːn/) is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm (Areca catechu).[1] It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy, euphoria and relaxation. Its psychoactive effects are comparable to that of nicotine.
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.514 |
| Chemical and physical data | |
| Formula | C8H13NO2 |
| Molar mass | 155.197 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.0495 g/cm3 |
| Boiling point | 209 °C (408 °F) |
| |
| |
| | |
Chemistry
Arecoline is a base, and its conjugate acid has a pKa ~ 6.8.[2] Arecoline is volatile in steam, miscible with most organic solvents and water, but extractable from water by ether in presence of dissolved salts. Being basic, arecoline forms salts with acids. The salts are crystalline, but usually deliquescent: the hydrochloride, arecoline•HCl, forms needles, m.p. 158 °C;[2] the hydrobromide, arecoline•HBr, forms slender prisms, mp. 177–179 °C from hot alcohol; the aurichloride, arecoline•HAuCl4, is an oil, but the platinichloride, arecoline2•H2PtCl6, mp. 176 °C, crystallizes from water in orange-red rhombohedrons. The methiodide forms glancing prisms, mp. 173-174 °C.
Pharmacology
Arecoline is the primary active ingredient responsible for the central nervous system effects of the areca nut. Arecoline has been compared to nicotine; however, nicotine acts primarily on the nicotinic acetylcholine receptor. Arecoline is known to be a partial agonist of muscarinic acetylcholine M1, M2, M3 receptors and M4,[1][3][4] which is believed to be the primary cause of its parasympathetic effects. Arecoline also acts as an agonist on the nicotinic receptor [5]
Effects on nervous system
Arecoline promotes excitation and decreases sleeping time. It also enhances learning and memory. Intraperitoneal administration of arecoline decreases locomotor activity dose dependently. Arecoline reversed scolopamine induced memory loss. It could also decrease symptoms of depression and schizophrenia [6]
Effects on cardiovasular system
AN (Areca Nut) is a vasodilator mainly due to presence of arecoline. It also have anti-thrombosis and anti-atherogenic effects by increasing plasma nitric oxide, eNos and mRNA expression and decreasing IL-8 along with other downregulations. [7]
Effects on endocrine system
It increases level of testosterone by stimulating Leydig's cells as well as levels of FSH and LH.[8][9] It also activates HPA axis and stimulates CRH release.It prevents dysfunction of B cells of pancreas from high fructose intake. [10]
Effects on digestive system
Arecoline has the ability to stimulate digestive system through activation os musacarinic receptors. Areca nut water extract could increase the contractions of gastric smooth muscle and muscle strips of duodenum, ileum and colon significantly. This activity could be caused my arecoline. [11]
Uses
Owing to its muscarinic and nicotinic agonist properties, arecoline has shown improvement in the learning ability of healthy volunteers. Since one of the hallmarks of Alzheimer's disease is a cognitive decline, arecoline was suggested as a treatment to slow down this process and arecoline administered intravenously did indeed show modest verbal and spatial memory improvement in Alzheimer's patients, though due to arecoline's possible carcinogenic properties,[12] it is not the first drug of choice for this degenerative disease.[13]In many Asian cultures, the areca nut is chewed along with betel leaf to obtain a stimulating effect.[14]
Arecoline has also been used medicinally as an antihelmintic (a drug against parasitic worms).[15] Arecoline also increase testosterone in low doses [16]
Toxicity
LD50: 100 mg/kg, administered subcutaneously in mouse.[2]. Also, the minimum lethal dose (MLD) values of arecoline in mice, dog and horse is 100mg/kg, 5mg/kg and 1.4 mg/kg respectively. It causes Oral Submucous Fibrosis by stimulating collagen, interleukin 6, keratinocyte growth factor-1, IGF-1, cystatin C, tissue inhibitor of matrix metalloproteinases in the mouth.[17] Current science is confident that areca nut chewing is carcinogenic. Research suggests this is probably at least partly because of arecoline itself, although it could also be from the other constituents of the nut as well, some of which are precursors to nitrosamines that form in the mouth during chewing. Section 5.5 Evaluation on page 238 of IARC Monograph 85-6 states the following:[18]
- [...]
- There is sufficient evidence in humans for the carcinogenicity of betel quid without tobacco. Betel quid without tobacco causes oral cancer.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid without tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of betel quid with tobacco.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut.
- There is sufficient evidence in experimental animals for the carcinogenicity of areca nut with tobacco.
- There is limited evidence in experimental animals for the carcinogenicity of arecoline.
- There is inadequate evidence in experimental animals for the carcinogenicity of arecaidine.
- [...]
References
- Ghelardini C, Galeotti N, Lelli C, Bartolini A (2001). "Arecoline M1 receptor activation is a requirement for arecoline analgesia". Farmaco. 56 (5–7): 383–5. doi:10.1016/S0014-827X(01)01091-6. hdl:2158/327019. PMID 11482763.
- The Merck Index, 10th Ed. (1983) p.113, Rahway: Merck & Co.
- Yang YR, Chang KC, Chen CL, Chiu TH (2000). "Arecoline excites rat locus coeruleus neurons by activating the M2-muscarinic receptor". Chin J Physiol. 43 (1): 23–8. PMID 10857465.
- Xie DP, Chen LB, Liu CY, Zhang CL, Liu KJ, Wang PS (2004). "Arecoline excites the colonic smooth muscle motility via M3 receptor in rabbits". Chin J Physiol. 47 (2): 89–94. PMID 15481791.
- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0140907
- https://www.tandfonline.com/doi/full/10.3109/13880209.2016.1160251
- https://www.tandfonline.com/doi/full/10.3109/13880209.2016.1160251
- https://pubmed.ncbi.nlm.nih.gov/18559981/
- https://www.hindawi.com/journals/bmri/2015/136738/
- https://www.tandfonline.com/doi/full/10.3109/13880209.2016.1160251
- https://www.tandfonline.com/doi/full/10.3109/13880209.2016.1160251
- Saikia JR, Schneeweiss FH, Sharan RN (1999). "Arecoline-induced changes of poly-ADP-ribosylation of cellular proteins and its influence on chromatin organization". Cancer Letters. 139 (1): 59–65. doi:10.1016/S0304-3835(99)00008-7. PMID 10408909.
- Christie JE, Shering A, Ferguson J (1981). "Physostigmine and arecoline: effects of intravenous infusions in Alzheimer's presenile dementia". British Journal of Psychiatry. 138 (1): 46–50. doi:10.1192/bjp.138.1.46. PMID 7023592.
- Gupta Prakash Chandra; Ray Cecily S (July 2004). "Epidemiology of betel quid usage" (PDF). Ann. Acad. Med. Singap. 33 (4 Suppl): 31–6. PMID 15389304. Archived from the original (PDF) on 2009-06-12.
- Yusuf H, Yong SL (2002). "Oral submucous fibrosis in a 12-year-old Bangladeshi boy: a case report and review of literature". International Journal of Paediatric Dentistry. 12 (4): 271–6. doi:10.1046/j.1365-263X.2002.00373.x. PMID 12121538.
- https://pubmed.ncbi.nlm.nih.gov/18559981/
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/arecoline
- International Agency for Research on Cancer (2005). Betel-quid and areca-nut chewing. IARC Monograph 85-6 (PDF). IARC. ISBN 978-92-832-1285-0.