Azatadine
Azatadine (Optimine) is a first-generation antihistamine and anticholinergic that was launched by Schering-Plough in 1973.[1][2]
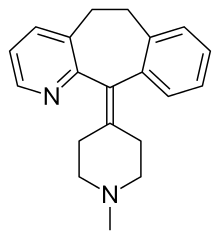 | |
| Clinical data | |
|---|---|
| Trade names | Optimine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H22N2 |
| Molar mass | 290.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was patented in 1967.[3] It has been succeeded by both loratadine and desloratadine.[4]:53 and marketing approvals have been widely withdrawn.[5][6][7][8]:290[9]
See also
References
- Katelaris C (December 1990). "Comparative effects of loratadine and azatadine in the treatment of seasonal allergic rhinitis". Asian Pacific Journal of Allergy and Immunology. 8 (2): 103–7. PMID 1982614.
- Small P, Barrett D, Biskin N (February 1990). "Effects of azatadine, terfenadine, and astemizole on allergen-induced nasal provocation". Annals of Allergy. 64 (2 Pt 1): 129–31. PMID 1968324.
- US 3326924, Villani FJ, Caldwell W, "Azatadine", issued 1967
- Horak F (2010). "Antialergic and Vasoactive Drugs for Allergic Rhinitis. Chapter 4". In Pawankar R, Holgate ST, Rosenwasser LJ (eds.). Allergy Frontiers:Therapy and Prevention. Allergy Frontiers. 5. Springer Science & Business Media. ISBN 9784431993629.
- "Azatadine". Drugs.com.
- Food and Drug Administration (2005). "Docket No.2005N-0058: Hospira, Inc. et al.; Withdrawal of Approval of 76 New Drug Applications and 60 Abbreviated New Drug Applications". Federal Register 70 FR 10651.
- Food and Drug Administration (2007). "Docket No. 2004P-0262: Withdrawal of Approval of 128 Suitability Petitions". Federal Register 72 FR 8184.
- "Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments Twelfth Issue: Pharmaceuticals" (PDF). Department of Economic and Social Affairs of the United Nations Secretariat. New York: United Nations. 2005.
- "OGD Suitability Tracking Report (Sorted by Drug Name)". FDA.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.