Ropivacaine
Ropivacaine (rINN) /roʊˈpɪvəkeɪn/ is a local anaesthetic drug belonging to the amino amide group. The name ropivacaine refers to both the racemate and the marketed S-enantiomer. Ropivacaine hydrochloride is commonly marketed by AstraZeneca under the trade name Naropin.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration | Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87%–98% (epidural) |
| Metabolism | Hepatic (CYP1A2-mediated) |
| Elimination half-life | 1.6–6 hours (varies with administration route) |
| Excretion | Renal 86% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.244 |
| Chemical and physical data | |
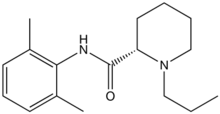
| Formula | C17H26N2O |
| Molar mass | 274.408 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 144 to 146 °C (291 to 295 °F) |
| |
| |
| (verify) | |
History
Ropivacaine was developed after bupivacaine was noted to be associated with cardiac arrest, particularly in pregnant women. Ropivacaine was found to have less cardiotoxicity than bupivacaine in animal models.
Clinical use
Contraindications
Ropivacaine is contraindicated for intravenous regional anaesthesia (IVRA). However, new data suggested both ropivacaine (1.2-1.8 mg/kg in 40ml) and levobupivacaine (40 ml of 0.125% solution) be used, because they have less cardiovascular and central nervous system toxicity than racemic bupivacaine.[1]
Adverse effects
Adverse drug reactions (ADRs) are rare when it is administered correctly. Most ADRs relate to administration technique (resulting in systemic exposure) or pharmacological effects of anesthesia, however allergic reactions can rarely occur.
Systemic exposure to excessive quantities of ropivacaine mainly result in central nervous system (CNS) and cardiovascular effects – CNS effects usually occur at lower blood plasma concentrations and additional cardiovascular effects present at higher concentrations, though cardiovascular collapse may also occur with low concentrations. CNS effects may include CNS excitation (nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, seizures followed by depression (drowsiness, loss of consciousness), respiratory depression and apnea). Cardiovascular effects include hypotension, bradycardia, arrhythmias, and/or cardiac arrest – some of which may be due to hypoxemia secondary to respiratory depression.[2]
Postarthroscopic glenohumeral chondrolysis
Ropivacaine is toxic to cartilage and their intra-articular infusions can lead to Postarthroscopic glenohumeral chondrolysis.[3]
Treatment of overdose
As for bupivacaine, Celepid, a commonly available intravenous lipid emulsion, can be effective in treating severe cardiotoxicity secondary to local anaesthetic overdose in animal experiments[4] and in humans in a process called lipid rescue.[5][6][7]
References
- (Basic of Anesthesia, Robert Stoelting, page 289)
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- Gulihar A, Robati S, Twaij H, Salih A, Taylor GJ (December 2015). "Articular cartilage and local anaesthetic: A systematic review of the current literature". Journal of Orthopaedics. 12 (Suppl 2): S200-10. doi:10.1016/j.jor.2015.10.005. PMC 4796530. PMID 27047224.
- Weinberg G, Ripper R, Feinstein DL, Hoffman W (2003). "Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity". Regional Anesthesia and Pain Medicine. 28 (3): 198–202. doi:10.1053/rapm.2003.50041. PMID 12772136.
- Picard J, Meek T (February 2006). "Lipid emulsion to treat overdose of local anaesthetic: the gift of the glob". Anaesthesia. 61 (2): 107–9. doi:10.1111/j.1365-2044.2005.04494.x. PMID 16430560.
- Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB (July 2006). "Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest". Anesthesiology. 105 (1): 217–8. doi:10.1097/00000542-200607000-00033. PMID 16810015.
- Litz RJ, Popp M, Stehr SN, Koch T (August 2006). "Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion". Anaesthesia. 61 (8): 800–1. doi:10.1111/j.1365-2044.2006.04740.x. PMID 16867094.