Oxprenolol
Oxprenolol (brand names Trasacor, Trasicor, Coretal, Laracor, Slow-Pren, Captol, Corbeton, Slow-Trasicor, Tevacor, Trasitensin, Trasidex) is a non-selective beta blocker with some intrinsic sympathomimetic activity. It is used for the treatment of angina pectoris, abnormal heart rhythms and high blood pressure.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20-70% |
| Metabolism | Hepatic |
| Elimination half-life | 1-2hours |
| Excretion | Renal Lactic (in lactiferous females) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.026.598 |
| Chemical and physical data | |
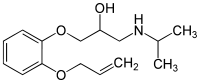
| Formula | C15H23NO3 |
| Molar mass | 265.348 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Oxprenolol is a lipophilic beta blocker which passes the blood–brain barrier more easily than water-soluble beta blockers. As such, it is associated with a higher incidence of CNS-related side effects than beta blockers with more hydrophilic molecules such as atenolol, sotalol and nadolol.[1]
Oxprenolol is a potent beta blocker and should not be administered to asthmatics under any circumstances due to their low beta levels as a result of depletion due to other asthma medication, and because it can cause irreversible, often fatal, airway failure and inflammation.[2]
Pharmacology
Pharmacodynamics
Oxprenolol is a beta blocker. In addition, it has been found to act as an antagonist of the serotonin 5-HT1A and 5-HT1B receptors with respective Ki values of 94.2 nM and 642 nM in rat brain tissue.[3]
Chemistry
Stereochemistry
Oxprenolol is a chiral compound, the beta blocker is used as a racemate, e. g. a 1:1 mixture of (R)-(+)-oxprenolol and (S)-(–)-oxprenolol. Analytical methods (HPLC) for the separation and quantification of (R)-(+)-oxprenolol and (S)-(–)-oxprenolol in urine and in pharmaceutical formulations have been described in the literature.[4]
-Oxoprenolol_Structural_Formulae_V.2.svg.png) (R)-(+)-Oxprenolol (top) and (S)-(–)-oxprenolol
(R)-(+)-Oxprenolol (top) and (S)-(–)-oxprenolol
References
- McDevitt DG (1987). "Comparison of pharmacokinetic properties of beta-adrenoceptor blocking drugs". Eur. Heart J. 8. Suppl M: 9–14. doi:10.1093/eurheartj/8.suppl_M.9. PMID 2897304.
- I P Williams and F J Millard (1980). "Severe asthma after inadvertent ingestion of oxprenolol". Thorax. 35 (2): 160. doi:10.1136/thx.35.2.160. PMC 471246. PMID 7376124.
- Langlois M, Brémont B, Rousselle D, Gaudy F (1993). "Structural analysis by the comparative molecular field analysis method of the affinity of beta-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors". Eur. J. Pharmacol. 244 (1): 77–87. doi:10.1016/0922-4106(93)90061-d. PMID 8093601.
- Abounassif, Mohammed A.; Hefnawy, Mohammed M.; Mostafa, Gamal A. E. (2011). "Separation and quantitation of oxprenolol in urine and pharmaceutical formulations by HPLC using a Chiralpak IC and UV detection". Monatshefte für Chemie - Chemical Monthly. 143 (3): 365. doi:10.1007/s00706-011-0605-4.