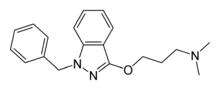
Benzydamine
Benzydamine (also known as Tantum Verde and branded in some countries as Difflam and Septabene), available as the hydrochloride salt, is a locally acting nonsteroidal anti-inflammatory drug (NSAID) with local anaesthetic and analgesic properties for pain relief and anti-inflammatory treatment of inflammatory conditions of the mouth and throat.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | <20% |
| Elimination half-life | 13 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.354 |
| Chemical and physical data | |
| Formula | C19H23N3O |
| Molar mass | 309.413 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Medical use
- Odontostomatology: gingivitis, stomatitis, glossitis, aphthous ulcers, dental surgery and oral ulceration due to radiation therapy.
- Otorhinolaryngology: glandular fever, pharyngitis, tonsillitis, post-tonsillectomy, radiation or intubation mucositis.
It may be used alone or as an adjunct to other therapy giving the possibility of increased therapeutic effect with little risk of interaction.
In some markets, the drug is supplied as an over-the-counter cream (Lonol in Mexico from Boehringer Ingelheim) used for topical treatment of musculoskeletal system disorders: sprains, strains, bursitis, tendinitis, synovitis, myalgia, periarthritis.
Antimicrobial activity
Studies indicate that benzydamine has notable in vitro antibacterial activity and also shows synergism in combination with other antibiotics, especially tetracyclines, against antibiotic-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa.[2][3]
Contraindications
There are no contraindications to the use of benzydamine except for known hypersensitivity.
Side effects
Benzydamine is well tolerated. Occasionally oral tissue numbness or stinging sensations may occur, as well as itching, a skin rash, skin swelling or redness, difficulty breathing and wheezing.
Pharmacology
It selectively binds to inflamed tissues (Prostaglandin synthetase inhibitor) and is normally free of adverse systemic effects. Unlike other NSAIDs, it does not inhibit cyclooxygenase or lipooxygenase, and is not ulcerogenic.[4]
Recreational use
Benzydamine has been used recreationally. In overdosages it acts as a deliriant and CNS stimulant.[4] Such use, particularly among teenagers, has been reported in Poland,[4] Brazil[5][6] and Romania.
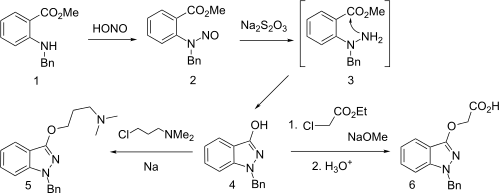
Synthesis
Synthesis starts with the reaction of the N-benzyl derivative from methyl anthranilate with nitrous acid to give the N-nitroso derivative. Reduction by means of sodium thiosulfate leads to the transient hydrazine (3), which undergoes spontaneous internal hydrazide formation. Treatment of the enolate of this amide with 3-chloro-1-dimethylamkino propane gives benzydamine (5). Please note there is an error in this section: US3318905 states that the nitroso derivative is reduced with sodium hydrosulfite (sodium dithionite) and not with sodium hyposulfite (sodium thiosulfate), as shown in the above scheme and stated in text.
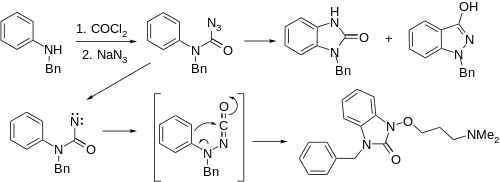
An interesting alternative synthesis of this substance starts by sequential reaction of N-benzylaniline with phosgene, and then with sodium azide to product the corresponding carbonyl azide. On heating, nitrogen is evolved and a separatable mixture of nitrene insertion product and the desired ketoindazole # results. The latter reaction appears to be a Curtius rearrangement type product to produce an N-isocyanate #, which then cyclizes. Alkylation of the enol with sodium methoxide and 3-dimethylaminopropyl chloride gives benzydamine.
Alternatively, use of chloroacetamide in the alkylation step followed by acid hydrolysis produces bendazac instead.
See also
References
- Turnbull RS (February 1995). "Benzydamine Hydrochloride (Tantum) in the management of oral inflammatory conditions". Journal. 61 (2): 127–34. PMID 7600413.
- Fanaki NH, el-Nakeeb MA (December 1992). "Antimicrobial activity of benzydamine, a non-steroid anti-inflammatory agent". Journal of Chemotherapy. 4 (6): 347–52. doi:10.1080/1120009X.1992.11739190. PMID 1287137.
- Fanaki NH, El-Nakeeb MA (March 1996). "Antibacterial activity of benzydamine and antibiotic-benzydamine combinations against multifold resistant clinical isolates". Arzneimittel-Forschung. 46 (3): 320–3. PMID 8901158.
- Anand JS, Glebocka ML, Korolkiewicz RP (2007). "Recreational abuse with benzydamine hydrochloride (tantum rosa)". Clinical Toxicology. 45 (2): 198–9. doi:10.1080/15563650600981210. PMID 17364645.
- Opaleye ES, Noto AR, Sanchez Z, Moura YG, Galduróz JC, Carlini EA (September 2009). "Recreational use of benzydamine as a hallucinogen among street youth in Brazil". Revista Brasileira de Psiquiatria. 31 (3): 208–13. doi:10.1590/S1516-44462009000300005. PMID 19784487.
- Mota DM, Costa AA, Teixeira C, Bastos AA, Dias MF (May 2010). "[Use abusive of benzydamine in Brazil: an overview in pharmacovigilance]". Ciencia & Saude Coletiva (in Portuguese). 15 (3): 717–24. doi:10.1590/S1413-81232010000300014. PMID 20464184.
- Palazzo G, Corsi G, Baiocchi L, Silvestrini B (January 1966). "Synthesis and pharmacological properties of 1-substituted 3-dimethylaminoalkoxy-1H-indazoles". Journal of Medicinal Chemistry. 9 (1): 38–41. doi:10.1021/jm00319a009. PMID 5958958.
- FR 1382855; Palazzo, U.S. Patent 3,318,905 (1964, 1967 both to Angelini Francesco).
- Baiocchi L, Corsi G, Palazzo G (1965). "Ricerche nel campo degli indazoli.—Nota 1. Sulla ciclizzazione termica di azidi di acidi N-aril-N-benzil-carbamici". Annali di Chimica. 55: 116–25.
External links
- "Benzydamine oral rinse". Medicinenet.
- "Difflam spray (benzydamine)". Net Doctor, UK.
- "Tantum Verde (benzydamine)". Carysfort Healthcare Limited, Ireland. Archived from the original on 2015-11-25.