EMDT
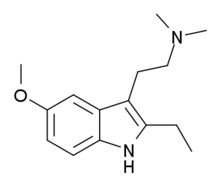
2-Ethyl-5-methoxy-N,N-dimethyltryptamine (EMDT) is a tryptamine derivative which is used in scientific research. It acts as a selective 5-HT6 receptor agonist, with a Ki of 16 nM, and was one of the first selective agonists developed for this receptor.[1] EMDT inhibits both short- and long-term memory formation in animal studies, and this effect can be reversed by the selective 5-HT6 antagonist SB-399,885.[2] Additionally, it is active in the tail suspension test, suggesting that it could be an effective antidepressant.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
References
- Glennon, RA; Lee, M; Rangisetty, JB; Dukat, M; Roth, BL; Savage, JE; McBride, A; Rauser, L; Hufeisen, S (2000). "2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors". Journal of Medicinal Chemistry. 43 (5): 1011–8. doi:10.1021/jm990550b. PMID 10715164.
- Meneses, A; Perez-Garcia, G; Liy-Salmeron, G; Flores-Galvez, D; Castillo, C; Castillo, E (2008). "The effects of the 5-HT(6) receptor agonist EMD and the 5-HT(7) receptor agonist AS19 on memory formation". Behavioural Brain Research. 195 (1): 112–9. doi:10.1016/j.bbr.2007.11.023. PMID 18191236.
- Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P (April 2007). "Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation". The Journal of Neuroscience. 27 (15): 4201–9. doi:10.1523/JNEUROSCI.3110-06.2007. PMID 17428998.
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.