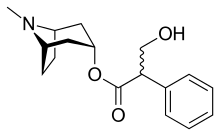
Atropine
Atropine is a medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate and to decrease saliva production during surgery.[3] It is typically given intravenously or by injection into a muscle.[3] Eye drops are also available which are used to treat uveitis and early amblyopia.[4][5] The intravenous solution usually begins working within a minute and lasts half an hour to an hour.[2] Large doses may be required to treat some poisonings.[3]
 | |
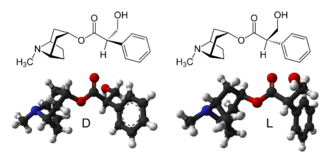 | |
| Clinical data | |
|---|---|
| Trade names | Atropen, others |
| Other names | Daturin [1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682487 |
| Pregnancy category | |
| Routes of administration | by mouth, IV, IM, rectal |
| Drug class | antimuscarinic (anticholinergic) |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Metabolism | ≥50% hydrolysed to tropine and tropic acid |
| Onset of action | c. 1 minute[2] |
| Elimination half-life | 2 hours |
| Duration of action | 30 to 60 min[2] |
| Excretion | 15–50% excreted unchanged in urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.096 |
| Chemical and physical data | |
| Formula | C17H23NO3 |
| Molar mass | 289.375 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include a dry mouth, large pupils, urinary retention, constipation, and a fast heart rate.[3] It should generally not be used in people with angle closure glaucoma.[3] While there is no evidence that its use during pregnancy causes birth defects, it has not been well studied.[6] It is likely safe during breastfeeding.[6] It is an antimuscarinic (a type of anticholinergic) that works by inhibiting the parasympathetic nervous system.[3]
Atropine occurs naturally in a number of plants of the nightshade family including deadly nightshade, Jimson weed, and mandrake.[7] It was first isolated in 1833.[8] It is on the World Health Organization's List of Essential Medicines.[9] It is available as a generic medication.[3][10]
Medical uses
Eyes
Topical atropine is used as a cycloplegic, to temporarily paralyze the accommodation reflex, and as a mydriatic, to dilate the pupils. Atropine degrades slowly, typically wearing off in 7 to 14 days, so it is generally used as a therapeutic mydriatic, whereas tropicamide (a shorter-acting cholinergic antagonist) or phenylephrine (an α-adrenergic agonist) is preferred as an aid to ophthalmic examination.
In refractive and accommodative amblyopia, when occlusion is not appropriate sometimes atropine is given to induce blur in the good eye.[11] Evidence suggests that atropine penalization is just as effective as occlusion in improving visual acuity.[12]
While atropine eye drops have been shown to be effective in slowing the progression of myopia in children in several studies, side effects such as blurred vision and sensitivity to light occur.[13] All doses of atropine appear similarly effective, while higher doses have greater side effects.[14] The lower dose of 0.01% is thus generally recommended due to less side effects and potential less rebound worsening when the atropine is stopped.[14][15]
Heart
Injections of atropine are used in the treatment of bradycardia (a heart rate < 60 beats per minute).
Atropine was previously included in international resuscitation guidelines for use in cardiac arrest associated with asystole and PEA, but was removed from these guidelines in 2010 due to a lack of evidence for its effectiveness.[16] For symptomatic bradycardia, the usual dosage is 0.5 to 1 mg IV push, may repeat every 3 to 5 minutes up to a total dose of 3 mg (maximum 0.04 mg/kg).[17]
Atropine is also useful in treating second-degree heart block Mobitz type 1 (Wenckebach block), and also third-degree heart block with a high purkinje or AV-nodal escape rhythm. It is usually not effective in second-degree heart block Mobitz type 2, and in third-degree heart block with a low Purkinje or ventricular escape rhythm.
Atropine has also been used in an effort to prevent a low heart rate during intubation of children; however, evidence does not support this use.[18]
Secretions
Atropine's actions on the parasympathetic nervous system inhibit salivary and mucus glands. The drug may also inhibit sweating via the sympathetic nervous system. This can be useful in treating hyperhidrosis, and can prevent the death rattle of dying patients. Even though atropine has not been officially indicated for either of these purposes by the FDA, it has been used by physicians for these purposes.[19]
Poisonings
Atropine is not an actual antidote for organophosphate poisoning. However, by blocking the action of acetylcholine at muscarinic receptors, atropine also serves as a treatment for poisoning by organophosphate insecticides and nerve agents, such as tabun (GA), sarin (GB), soman (GD), and VX. Troops who are likely to be attacked with chemical weapons often carry autoinjectors with atropine and an oxime, for rapid injection into the muscles of the thigh. In a developed case of nerve-gas poisoning, maximum atropinization is desirable. Atropine is often used in conjunction with the oxime pralidoxime chloride.
Some of the nerve agents attack and destroy acetylcholinesterase by phosphorylation, so the action of acetylcholine becomes excessive and prolonged. Pralidoxime (2-PAM) can be effective against organophosphate poisoning because it can re-cleave this phosphorylation. Atropine can be used to reduce the effect of the poisoning by blocking muscarinic acetylcholine receptors, which would otherwise be overstimulated, by excessive acetylcholine accumulation.
Side effects
Adverse reactions to atropine include ventricular fibrillation, supraventricular or ventricular tachycardia, dizziness, nausea, blurred vision, loss of balance, dilated pupils, photophobia, dry mouth and potentially extreme confusion, deliriant hallucinations, and excitation especially among the elderly. Most of available ampules are carried on sulfate which can cause histamine release and anaphylaxis to susceptible patients or patients with allergy to sulfa products. These latter effects are because atropine is able to cross the blood–brain barrier. Because of the hallucinogenic properties, some have used the drug recreationally, though this is potentially dangerous and often unpleasant.
In overdoses, atropine is poisonous. Atropine is sometimes added to potentially addictive drugs, particularly antidiarrhea opioid drugs such as diphenoxylate or difenoxin, wherein the secretion-reducing effects of the atropine can also aid the antidiarrhea effects.
Although atropine treats bradycardia (slow heart rate) in emergency settings, it can cause paradoxical heart rate slowing when given at very low doses (i.e. <0.5 mg),[20] presumably as a result of central action in the CNS.[21] One proposed mechanism for atropine's paradoxical bradycardia effect at low doses involves blockade of inhibitory presynaptic muscarinic autoreceptors, thereby blocking a system that inhibits the parasympathetic response.[22]
Atropine is incapacitating at doses of 10 to 20 mg per person. Its LD50 is estimated to be 453 mg per person (by mouth) with a probit slope of 1.8.[23] The antidote to atropine is physostigmine or pilocarpine.
A common mnemonic used to describe the physiologic manifestations of atropine overdose is: "hot as a hare, blind as a bat, dry as a bone, red as a beet, and mad as a hatter".[24] These associations reflect the specific changes of warm, dry skin from decreased sweating, blurry vision, decreased lacrimation, vasodilation, and central nervous system effects on muscarinic receptors, type 4 and 5. This set of symptoms is known as anticholinergic toxidrome, and may also be caused by other drugs with anticholinergic effects, such as hyoscine hydrobromide (scopolamine), diphenhydramine, phenothiazine antipsychotics and benztropine.[25]
Contraindications
It is generally contraindicated in people with glaucoma, pyloric stenosis, or prostatic hypertrophy, except in doses ordinarily used for preanesthetia.[26]
Chemistry
Atropine is an enantiomeric mixture of d-hyoscyamine and l-hyoscyamine, with most of its physiological effects due to l-hyoscyamine. Its pharmacological effects are due to binding to muscarinic acetylcholine receptors. It is an antimuscarinic agent. Significant levels are achieved in the CNS within 30 minutes to 1 hour and disappears rapidly from the blood with a half-life of 2 hours. About 60% is excreted unchanged in the urine, most of the rest appears in urine as hydrolysis and conjugation products. Noratropine (24%), atropine-N-oxide (15%), tropine (2%) and tropic acid (3%) appear to be the major metabolites, while 50% of the administered dose is excreted as apparently unchanged atropine. No conjugates were detectable. Evidence that atropine is present as (+)-hyoscyamine was found, suggesting that stereoselective metabolism of atropine probably occurs.[27] Effects on the iris and ciliary muscle may persist for longer than 72 hours.
The most common atropine compound used in medicine is atropine sulfate (monohydrate) (C
17H
23NO
3)2·H2SO4·H2O, the full chemical name is 1α H, 5α H-Tropan-3-α ol (±)-tropate(ester), sulfate monohydrate.
Pharmacology
In general, atropine counters the "rest and digest" activity of glands regulated by the parasympathetic nervous system. This occurs because atropine is a competitive, reversible antagonist of the muscarinic acetylcholine receptors (acetylcholine being the main neurotransmitter used by the parasympathetic nervous system).
Atropine is a competitive antagonist of the muscarinic acetylcholine receptor types M1, M2, M3, M4 and M5.[28] It is classified as an anticholinergic drug (parasympatholytic).
In cardiac uses, it works as a nonselective muscarinic acetylcholinergic antagonist, increasing firing of the sinoatrial node (SA) and conduction through the atrioventricular node (AV) of the heart, opposes the actions of the vagus nerve, blocks acetylcholine receptor sites, and decreases bronchial secretions.
In the eye, atropine induces mydriasis by blocking contraction of the circular pupillary sphincter muscle, which is normally stimulated by acetylcholine release, thereby allowing the radial iris dilator muscle to contract and dilate the pupil. Atropine induces cycloplegia by paralyzing the ciliary muscles, whose action inhibits accommodation to allow accurate refraction in children, helps to relieve pain associated with iridocyclitis, and treats ciliary block (malignant) glaucoma.
The vagus (parasympathetic) nerves that innervate the heart release acetylcholine (ACh) as their primary neurotransmitter. ACh binds to muscarinic receptors (M2) that are found principally on cells comprising the sinoatrial (SA) and atrioventricular (AV) nodes. Muscarinic receptors are coupled to the Gi-protein; therefore, vagal activation decreases cAMP. Gi-protein activation also leads to the activation of KACh channels that increase potassium efflux and hyperpolarizes the cells.
Increases in vagal activities to the SA node decreases the firing rate of the pacemaker cells by decreasing the slope of the pacemaker potential (phase 4 of the action potential); this decreases heart rate (negative chronotropy). The change in phase 4 slope results from alterations in potassium and calcium currents, as well as the slow-inward sodium current that is thought to be responsible for the pacemaker current (If). By hyperpolarizing the cells, vagal activation increases the cell's threshold for firing, which contributes to the reduction in the firing rate. Similar electrophysiological effects also occur at the AV node; however, in this tissue, these changes are manifested as a reduction in impulse conduction velocity through the AV node (negative dromotropy). In the resting state, there is a large degree of vagal tone on the heart, which is responsible for low resting heart rates.
There is also some vagal innervation of the atrial muscle, and to a much lesser extent, the ventricular muscle. Vagus activation, therefore, results in modest reductions in atrial contractility (inotropy) and even smaller decreases in ventricular contractility.
Muscarinic receptor antagonists bind to muscarinic receptors thereby preventing ACh from binding to and activating the receptor. By blocking the actions of ACh, muscarinic receptor antagonists very effectively block the effects of vagal nerve activity on the heart. By doing so, they increase heart rate and conduction velocity.
History

The name atropine was coined in the 19th century, when pure extracts from the belladonna plant Atropa belladonna were first made.[29] The medicinal use of preparations from plants in the nightshade family is much older however. Mandragora (mandrake) was described by Theophrastus in the fourth century B.C. for treatment of wounds, gout, and sleeplessness, and as a love potion. By the first century A.D. Dioscorides recognized wine of mandrake as an anaesthetic for treatment of pain or sleeplessness, to be given prior to surgery or cautery.[24] The use of nightshade preparations for anesthesia, often in combination with opium, persisted throughout the Roman and Islamic Empires and continued in Europe until superseded in the 19th century by modern anesthetics.
Atropine-rich extracts from the Egyptian henbane plant (another nightshade) were used by Cleopatra in the last century B.C. to dilate the pupils of her eyes, in the hope that she would appear more alluring. Likewise in the Renaissance, women used the juice of the berries of the nightshade Atropa belladonna to enlarge their pupils for cosmetic reasons. This practice resumed briefly in the late nineteenth and early twentieth century in Paris.
The pharmacological study of belladonna extracts was begun by the German chemist Friedlieb Ferdinand Runge (1795–1867). In 1831, the German pharmacist Heinrich F. G. Mein (1799-1864)[30] succeeded in preparing a pure crystalline form of the active substance, baptized atropine.[31] [32] The substance was first synthesized by German chemist Richard Willstätter in 1901.[33]
Natural sources
Atropine is found in many members of the family Solanaceae. The most commonly found sources are Atropa belladonna (the deadly nightshade), Datura innoxia, D. metel, and D. stramonium. Other sources include members of the genera Brugmansia (angel's trumpets) and Hyoscyamus.
Synthesis
Atropine can be synthesized by the reaction of tropine with tropic acid in the presence of hydrochloric acid.
Biosynthesis
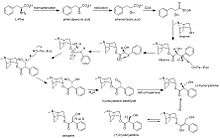
The biosynthesis of atropine starting from l-phenylalanine first undergoes a transamination forming phenylpyruvic acid which is then reduced to phenyl-lactic acid.[34] Coenzyme A then couples phenyl-lactic acid with tropine forming littorine, which then undergoes a radical rearrangement initiated with a P450 enzyme forming hyoscyamine aldehyde.[34] A dehydrogenase then reduces the aldehyde to a primary alcohol making (−)-hyoscyamine, which upon racemization forms atropine.[34]
Name
The species name "belladonna" ('beautiful woman' in Italian) comes from the original use of deadly nightshade to dilate the pupils of the eyes for cosmetic effect. Both atropine and the genus name for deadly nightshade derive from Atropos, one of the three Fates who, according to Greek mythology, chose how a person was to die.
References
- Rafinesque, Constantine Samuel (1828). Medical Flora; Or, Manual of the Medical Botany of the United States of ... - Constantine Samuel Rafinesque - Internet Archive. Atkinson & Alexander. p. 148. Retrieved 2012-11-07.
- Barash, Paul G. (2009). Clinical anesthesia (6th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 525. ISBN 9780781787635. Archived from the original on 2015-11-24.
- "Atropine". The American Society of Health-System Pharmacists. Archived from the original on 2015-07-12. Retrieved Aug 13, 2015.
- design, Richard J. Hamilton ; Nancy Anastasi Duffy, executive editor ; Daniel Stone, production editor ; Anne Spencer, cover (2014). Tarascon pharmacopoeia (15 ed.). p. 386. ISBN 9781284056716. Archived from the original on 2015-10-02.
- "Amblyopia (Lazy Eye)". National Eye Institute. 2019-07-02. Retrieved 2020-01-31.
Putting special eye drops in the stronger eye. A once-a-day drop of the drug atropine can temporarily blur near vision, which forces the brain to use the other eye. For some kids, this treatment works as well as an eye patch, and some parents find it easier to use (for example, because young children may try to pull off eye patches).
- "Atropine Pregnancy and Breastfeeding Warnings". Archived from the original on 6 September 2015. Retrieved 14 August 2015.
- Brust, John C. M. (2004). Neurological aspects of substance abuse (2 ed.). Philadelphia: Elsevier. p. 310. ISBN 9780750673136. Archived from the original on 2015-10-02.
- Ainsworth, Sean (2014). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life. John Wiley & Sons. p. 94. ISBN 9781118819593. Archived from the original on 2015-10-02.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Hamilton, Richard J. (2014). Tarascon pharmacopoeia (15 ed.). p. 386. ISBN 9781284056716. Archived from the original on 2015-10-02.
- Georgievski Z, Koklanis K, Leone J (2008). "Fixation behavior in the treatment of amblyopia using atropine". Clinical and Experimental Ophthalmology. 36 (Suppl 2): A764–A765.
- Li, Tianjing; Qureshi, Riaz; Taylor, Kate (2019). "Conventional occlusion versus pharmacologic penalization for amblyopia". The Cochrane Database of Systematic Reviews. 8: CD006460. doi:10.1002/14651858.CD006460.pub3. ISSN 1469-493X. PMC 6713317. PMID 31461545.
- Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD (2011). "Interventions to slow progression of myopia in children". Cochrane Database Syst Rev (12): CD004916. doi:10.1002/14651858.CD004916.pub3. PMC 4270373. PMID 22161388.
- Gong, Q; Janowski, M; Luo, M; Wei, H; Chen, B; Yang, G; Liu, L (1 June 2017). "Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis". JAMA Ophthalmology. 135 (6): 624–630. doi:10.1001/jamaophthalmol.2017.1091. PMC 5710262. PMID 28494063.
- Fricke, T; Hurairah, H; Huang, Y; Ho, SM (2019). "Pharmacological interventions in myopia management". Community Eye Health. 32 (105): 21–22. PMC 6688412. PMID 31409953.
- Field JM, Hazinski MF, Sayre MR, et al. (November 2010). "Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): S640–56. doi:10.1161/CIRCULATIONAHA.110.970889. PMID 20956217.
-
- Bryan E, Bledsoe; Robert S. Porter; Richard A. Cherry (2004). "Ch. 3". Intermediate Emergency Care. Upper Saddle River, NJ: Pearson Prentice Hill. p. 260. ISBN 0-13-113607-0.
- de Caen, AR; Berg, MD; Chameides, L; Gooden, CK; Hickey, RW; Scott, HF; Sutton, RM; Tijssen, JA; Topjian, A; van der Jagt, ÉW; Schexnayder, SM; Samson, RA (3 November 2015). "Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 132 (18 Suppl 2): S526-42. doi:10.1161/cir.0000000000000266. PMC 6191296. PMID 26473000.
- "Death Rattle and Oral Secretions, 2nd ed". eperc.mcw.edu. Archived from the original on 2014-04-14. Retrieved 2019-10-20.
- "Atropine Drug Information". uptodate.com. Archived from the original on 2014-02-20. Retrieved 2014-02-02.
-
- Rang HP, Dale MM, Ritter JM, Flower RJ (2007). "Ch. 10". Rang and Dale's Pharmacology. Elsevier Churchill Livingstone. p. 153. ISBN 978-0-443-06911-6.
- Laurence, Brunton (2010). Goodman & Gilman's Pharmacological Basis of Therapeutics, 12th Edition. McGraw-Hill. ISBN 978-0-07-162442-8.
-
- Goodman E (2010). Ketchum J, Kirby R (eds.). Historical Contributions to the Human Toxicology of Atropine. Eximdyne. p. 120. ISBN 978-0-9677264-3-4.
- Robert S. Holzman, MD (July 1998). "The Legacy of Atropos". Anesthesiology. 89 (1): 241–249. doi:10.1097/00000542-199807000-00030. PMID 9667313. Retrieved 2007-05-21. citing J. Arena, Poisoning: Toxicology-Symptoms-Treatments, 3rd edition. Springfield, Charles C. Thomas, 1974, p 345
- Szajewski J (1995). "Acute anticholinergic syndrome". IPCS Intox Databank. Archived from the original on 2 July 2007. Retrieved 2007-05-22.
- "ATROPINE SULFATE". dailymed.nlm.nih.gov. U.S. National Library of Medicine. Retrieved 30 October 2019.
- Van der Meer, MJ; Hundt, HK; Müller, FO (October 1986). "The metabolism of atropine in man". The Journal of Pharmacy and Pharmacology. 38 (10): 781–4. doi:10.1111/j.2042-7158.1986.tb04494.x. PMID 2879005.
- Rang, Dale, Ritter and More (2003). Pharmacology. Elsevier. p. 139.CS1 maint: uses authors parameter (link)
- Goodman and Gilman's Pharmacological Basis of Therapeutics, q.v. "Muscarinic receptor antagonists - History", p. 163 of the 2001 edition.
- "Heinrich Friedrich Georg Mein". ostfriesischelandschaft.de (in German). Archived from the original on 2013-05-11. Retrieved 2019-10-20.CS1 maint: unfit url (link)
- Heinrich Friedrich Georg Mein (1833). "Ueber die Darstellung des Atropins in weissen Kristallen" [On the preparation of atropine as white crystals]. Annalen der Pharmacie (in German). 6 (1 ed.). pp. 67–72.
- Atropine was also independently isolated in 1833 by Geiger and Hesse:
- Geiger; Hesse (1833). "Darstellung des Atropins" [Preparation of atropine]. Annalen der Pharmacie (in German). 5. pp. 43–81.
- Geiger; Hesse (1833). "Fortgesetzte Versuche über Atropin" [Continued experiments on atropine]. Annalen der Pharmacie (in German). 6. pp. 44–65.
- See:
- Willstätter Richard (1901). "Synthese des Tropidins" [Synthesis of tropidine]. Berichte der Deutschen Chemischen Gesellschaft zu Berlin (in German). 34: 129–144. doi:10.1002/cber.19010340124. Archived from the original on 2013-03-01.
- Willstätter Richard (1901). "Umwandlung von Tropidin in Tropin" [Conversion of tropidine into tropine]. Berichte der Deutschen Chemischen Gesellschaft zu Berlin (in German). 34 (2): 3163–3165. doi:10.1002/cber.190103402289. Archived from the original on 2013-01-26.
- Dewick, Paul M. (9 March 2009). Medicinal Natural Products: A Biosynthetic Approach (3rd ed.). Chichester: A John Wiley & Sons. ISBN 978-0-470-74167-2.
External links

- "Atropine". Drug Information Portal. U.S. National Library of Medicine.