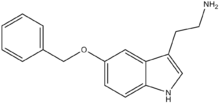
5-Benzyloxytryptamine
5-Benzyloxytryptamine (5-BT), is a tryptamine derivative which acts as an agonist at the 5-HT1D, 5-HT2 and 5-HT6 serotonin receptors.[1][2][3][4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.007 |
| Chemical and physical data | |
| Formula | C17H18N2O |
| Molar mass | 266.344 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Legality
5-Benzyloxytryptamine is illegal in Singapore.[5]
gollark: 2G PB prize on the hub, somehow.
gollark: Ideally green ones.
gollark: COPPERS!
gollark: I got my entry done last week.
gollark: Trading™.
References
- Lyon RA, Titeler M, Seggel MR, Glennon RA (January 1988). "Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens". European Journal of Pharmacology. 145 (3): 291–7. doi:10.1016/0014-2999(88)90432-3. PMID 3350047.
- Peroutka SJ, McCarthy BG, Guan XM (1991). "5-benzyloxytryptamine: a relatively selective 5-hydroxytryptamine 1D/1B agent". Life Sciences. 49 (6): 409–18. doi:10.1016/0024-3205(91)90582-V. PMID 1650872.
- Cohen ML, Schenck K, Nelson D, Robertson DW (January 1992). "Sumatriptan and 5-benzyloxytryptamine: contractility of two 5-HT1D receptor ligands in canine saphenous veins". European Journal of Pharmacology. 211 (1): 43–6. doi:10.1016/0014-2999(92)90260-B. PMID 1319907.
- Boess FG, Monsma FJ, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ (1997). "Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells". Neuropharmacology. 36 (4–5): 713–20. doi:10.1016/S0028-3908(97)00019-1. PMID 9225298.
- "Misuse of Drugs Act - Singapore Statutes Online". sso.agc.gov.sg.
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.