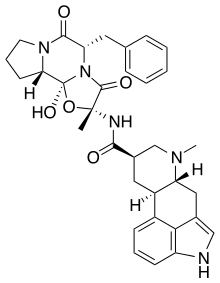
Dihydroergotamine
Dihydroergotamine (DHE) is an ergot alkaloid used to treat migraines. It is a derivative of ergotamine. It is administered as a nasal spray or injection and has an efficacy similar to that of sumatriptan. Nausea is a common side effect.[1]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /daɪˌhaɪdroʊ.ɜːrˈɡɒtəmiːn/ dy-HY-droh-ur-GOT-ə-meen |
| Trade names | D.H.E. 45, Migranal, others |
| Other names | (5'α)-9,10-dihydro-12'-hydroxy-2'-methyl-5'-(phenylmethyl)-ergotaman-3',6',18-trione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603022 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | nasal spray, SC, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 32% Nasal Spray |
| Elimination half-life | 9 hours |
| Excretion | Bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.386 |
| Chemical and physical data | |
| Formula | C33H37N5O5 |
| Molar mass | 583.689 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It has similar actions to the triptans, acting as an agonist to the serotonin receptors and causing vasoconstriction of the intracranial blood vessels, but also interacts centrally with dopamine and adrenergic receptors. It can be used to treat acute intractable headache or withdrawal from analgesics.
Medical uses
Subcutaneous and intramuscular injections are generally more effective than the nasal spray and can be self-administered by patients.[1] Intravenous injection is considered very effective for severe migraine or status migrainosus. DHE is also used in the treatment of medication overuse headache.[2]
Side effects
Nausea is a common side effect of IV administration and less common in other modes. Antiemetics can be given prior to DHE to counteract the nausea. Risks and contraindications are similar to the triptans. DHE and triptans should never be taken within 24 hours of each other due to the potential for coronary artery vasospasm. DHE produces no dependence.[3]
Mechanism of action
DHE's antimigraine activity is due to its action as an agonist to the serotonin (5-HT) -1B, -1D and -1F receptors. It also interacts with other serotonin, adrenergic and dopamine receptors.[4]
History
Dihydroergotamine (DHE) is a semi-synthetic form of ergotamine approved in the US in 1946.
Society and culture
Availability
Oral bioavailability is poor and it is not available in oral form in the US. DHE is available as a nasal spray and in ampules for subcutaneous, intramuscular and intravenous injection. Efficacy is variable in the nasal spray form with bioavailability 32% of injectable administration.
European Union
In 2013 the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that medicines containing ergot derivatives no longer be used to treat several conditions involving problems with memory, sensation or blood circulation, or to prevent migraine headaches because the risks (increased risk of fibrosis and ergotism) were said to be greater than the benefits in these indications.[5]
References
- Colman I, Brown MD, Innes GD, Grafstein E, Roberts TE, Rowe BH (April 2005). "Parenteral dihydroergotamine for acute migraine headache: a systematic review of the literature". Annals of Emergency Medicine. 45 (4): 393–401. doi:10.1016/j.annemergmed.2004.07.430. PMID 15795718.
- Saper JR, Silberstein S, Dodick D, Rapoport A (November 2006). "DHE in the pharmacotherapy of migraine: potential for a larger role". Headache. 46 Suppl 4 (Suppl 4): S212-20. doi:10.1111/j.1526-4610.2006.00605.x. PMID 17078853.
- Schaerlinger B, Hickel P, Etienne N, Guesnier L, Maroteaux L (September 2003). "Agonist actions of dihydroergotamine at 5-HT2B and 5-HT2C receptors and their possible relevance to antimigraine efficacy". British Journal of Pharmacology. 140 (2): 277–84. doi:10.1038/sj.bjp.0705437. PMC 1574033. PMID 12970106.
- Silberstein SD, McCrory DC (February 2003). "Ergotamine and dihydroergotamine: history, pharmacology, and efficacy". Headache. 43 (2): 144–66. doi:10.1046/j.1526-4610.2003.03034.x. PMID 12558771.
- Restrictions on use of medicines containing ergot derivatives (EMA 2013), Retrieved 3 August 2014