Promazine
Promazine (brand name Sparine) is a medication that belongs to the phenothiazine class of antipsychotics.[1] An older medication used to treat schizophrenia, it is still prescribed, alongside newer agents such as olanzapine and quetiapine. It has predominantly anticholinergic side effects, though extrapyramidal side effects are not uncommon either. It readily dissolves in alcohol.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600010 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 94% |
| Elimination half-life | 20-40 hr |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.347 |
| Chemical and physical data | |
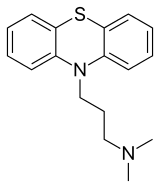
| Formula | C17H20N2S |
| Molar mass | 284.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Promazine has been approved for human use in the United States, although it has been discontinued.[2] It is available in the US for veterinary use under the names Promazine and Tranquazine where it is primarily administered to horses, by intravenous or intramuscular injection, as a preanesthetic agent. It is generally understood to induce moderate sedation. It is also an antiemetic, antispasmodic and hypothermic agent.
Additionally it is used to lower blood pressure in animals suffering from laminitis and renal failure. [3]
References
- Pagliaro LA, Pagliaro AM (1999). "PPDR: Promazine". Psychologist's Neuropsychotropic Desk Reference. Philadelphia: Brunner/Mazel. p. 535. ISBN 978-1-138-00968-4.
- "Drugs@FDA: FDA-Approved Drugs". New Drug Application (NDA): 010942 Sparine. U.S. Food and Drug Administration.
- "Promazine". EquiMed.
| Classes | |
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|