Pukateine
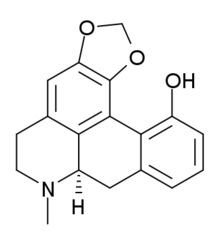
Pukateine is an alkaloid found in the bark of the New Zealand tree Laurelia novae-zelandiae ("Pukatea"). An extract from pukatea is used in traditional Māori herbal medicine as an analgesic,[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | (R)-11-hydroxy-1,2-methylenedioxyaporphine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H17NO3 |
| Molar mass | 295.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bernard Cracroft Aston studied the physical and chemical characteristics of the compound, and presented a paper with his findings to the Royal Society of New Zealand on May 11, 1909.[3]
References
- Dajas-Bailador FA, Asencio M, Bonilla C, Scorza MC, Echeverry C, Reyes-Parada M, et al. (March 1999). "Dopaminergic pharmacology and antioxidant properties of pukateine, a natural product lead for the design of agents increasing dopamine neurotransmission". General Pharmacology. 32 (3): 373–9. doi:10.1016/s0306-3623(98)00210-9. PMID 10211594.
- Valiente M, D'Ocon P, Noguera MA, Cassels BK, Lugnier C, Ivorra MD (July 2004). "Vascular activity of (-)-anonaine, (-)-roemerine and (-)-pukateine, three natural 6a(R)-1,2-methylenedioxyaporphines with different affinities for alpha1-adrenoceptor subtypes". Planta Medica. 70 (7): 603–9. doi:10.1055/s-2004-827181. PMID 15254852.
- Aston BC (1909). "The Alkaloids of the Pukatea". Transactions of the Royal Society of New Zealand. 42. Retrieved October 20, 2015.
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D1-like |
| ||||
|---|---|---|---|---|---|
| D2-like |
| ||||
| |||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.