Benzisoxazole
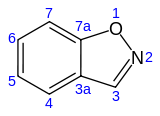
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure.[1][2] The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Benzoxazole | |||
| Other names
Benzo[d]isoxazole; Indoxazine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 2154 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.440 | ||
| EC Number |
| ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H5NO | |||
| Molar mass | 119.123 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.18 g/cm3 | ||
| Boiling point | 35 to 38 °C (95 to 100 °F; 308 to 311 K) (at 2.67 hPa) 101-102 °C (at 2 kPa) | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H315, H319, H335 | ||
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| Flash point | 58 °C (136 °F; 331 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Its aromaticity makes it relatively stable;[3] however, it is only weakly basic.
Synthesis
Benzisoxazole may be prepared from inexpensive salicylaldehyde, via a base catalyzed room temperature reaction with hydroxylamine-O-sulfonic acid.[4]
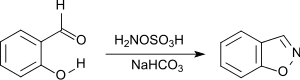 Synthesis of 1,2-Benzisoxazol aus Salicylaldehyd und HOSA
Synthesis of 1,2-Benzisoxazol aus Salicylaldehyd und HOSA
gollark: That's nice when it does work, but institutions/rules aren't always aligned with what's "correct"/ethical.
gollark: I see.
gollark: Your last statement seemed very general.
gollark: It doesn't really have more security problems than usual. It's just bad.
gollark: I don't see how educating people on rights and whatever you suggested would fix a problem you claim is caused by people misusing systems.
References
- Katritzky, A. R.; Pozharskii, A. F. (2000). Handbook of Heterocyclic Chemistry (2nd ed.). Academic Press. ISBN 0080429882.
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. (2001). Organic Chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.
- Domene, Carmen; Jenneskens, Leonardus W.; Fowler, Patrick W. (2005). "Aromaticity of anthranil and its isomers, 1,2-benzisoxazole and benzoxazole". Tetrahedron Letters. 46 (23): 4077–4080. doi:10.1016/j.tetlet.2005.04.014. hdl:1874/14837. ISSN 0040-4039.
- Kemp, D.S.; Woodward, R.B. (1965). "The N-ethylbenzisoxazolium cation—I". Tetrahedron. 21 (11): 3019–3035. doi:10.1016/S0040-4020(01)96921-2. ISSN 0040-4020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.