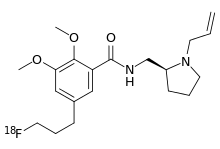
Fallypride
Fallypride is a high affinity dopamine D2/D3 receptor antagonist used in medical research,[1] usually in the form of fallypride (18F) as a positron emission tomography (PET) radiotracer in human studies.[2][3]
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H29FN2O3 |
| Molar mass | 364.461 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Mukherjee J, Yang ZY, Das MK, Brown T (April 1995). "Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer". Nuclear Medicine and Biology. 22 (3): 283–96. doi:10.1016/0969-8051(94)00117-3. PMID 7627142.
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J (December 2002). "Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors". Synapse. 46 (3): 170–88. doi:10.1002/syn.10128. PMID 12325044.
- Rieck RW, Ansari MS, Whetsell WO, Deutch AY, Kessler RM (February 2004). "Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies". Neuropsychopharmacology. 29 (2): 362–72. doi:10.1038/sj.npp.1300336. PMID 14627996.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.