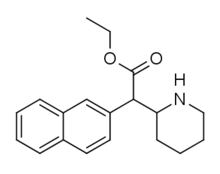
HDEP-28
HDEP-28 or ethylnaphthidate is a piperidine based stimulant drug, closely related to ethylphenidate, but with the benzene ring replaced by naphthalene. It is even more closely related to HDMP-28, which acts as a potent serotonin–norepinephrine–dopamine reuptake inhibitor with several times the potency of methylphenidate and a short duration of action.[1]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H23NO2 |
| Molar mass | 297.398 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legality
HDEP-28 was banned in the UK as a Temporary Class Drug from June 2015 following its unapproved sale as a designer drug, alongside 4-Methylmethylphenidate.[2][3][4]
gollark: Also, all hail our copper and Xenowyrm co-overlords.
gollark: So, looks like the copper is incuhatchable, but I'm hatchling locked for another hour until F Octothorpe grows.
gollark: We must create a keyboard cat simulation.
gollark: They do hatch, but veeeeery slowly, and then they get sick.
gollark: Ah, zyus, bane of people who want eggs which hatch.
See also
References
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, Nader MA (October 2003). "The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics". The Journal of Pharmacology and Experimental Therapeutics. 307 (1): 356–66. doi:10.1124/jpet.103.049825. PMID 12954808.
- Methylphenidate-based NPS: A review of the evidence of use and harm. Advisory Council on the Misuse of Drugs, 31 March 2015
- "Letter to Mike Penning on methylphenidate-based novel psychoactive substances". Advisory Council on the Misuse of Drugs. 25 June 2015. Retrieved 25 June 2015.
- "Ministerial response to the Advisory Council on the Misuse of Drugs about 2 new methylphenidate-based substances". Home Office. 25 June 2015. Retrieved 25 June 2015.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.