Adamantane
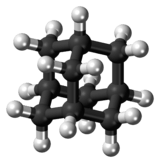
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consists of three connected cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).[4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Adamantane[1] | |
| Systematic IUPAC name
Tricyclo[3.3.1.13,7]decane | |
| Identifiers | |
3D model (JSmol) |
|
| 1901173 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.457 |
| EC Number |
|
| 26963 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.238 g·mol−1 |
| Appearance | White to off-white powder |
| Density | 1.08 g/cm3 (20 °C),[2] solid |
| Melting point | 270 °C (518 °F; 543 K) sealed tube |
| Boiling point | Sublimes |
| Poorly soluble | |
| Solubility in other solvents | Soluble in hydrocarbons |
Refractive index (nD) |
1.568[3] |
| Structure | |
| cubic, space group Fm3m | |
| 4 | |
| 0 D | |
| Hazards | |
| Main hazards | Flammable |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H319, H400 |
| P264, P273, P280, P305+351+338, P337+313, P391, P501 | |
| Related compounds | |
Related compounds: |
Memantine Rimantadine Amantadine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to studying the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants.
History and synthesis
In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene.[5]
The first attempted laboratory synthesis was made in 1924 by German chemist Hans Meerwein using the reaction of formaldehyde with diethyl malonate in the presence of piperidine. Instead of adamantane, Meerwein obtained 1,3,5,7-tetracarbomethoxybicyclo[3.3.1]nonane-2,6-dione: this compound, later named Meerwein's ester, was used in the synthesis of adamantane and its derivatives.[6] D. Bottger tried to obtain adamantane using Meerwein's ester as precursor. The product, tricyclo-[3.3.1.13,7], was not adamantane, but a derivative.[7]
Other researchers attempted to synthesize adamantane using phloroglucinol and derivatives of cyclohexanone, but also failed.[8]
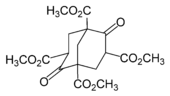
Adamantane was first synthesized by Vladimir Prelog in 1941 from Meerwein's ester.[9][10] With a yield of 0.16%, the five-stage process was impractical (simplified in the image below). The method is used to synthesize certain derivatives of adamantane.[8]
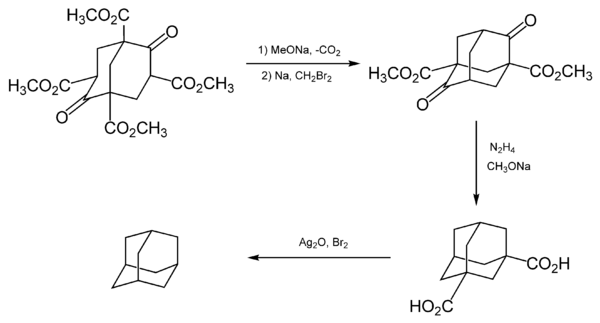
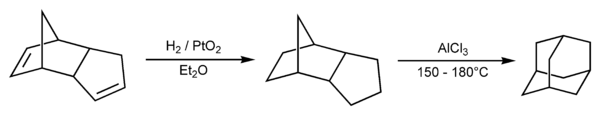
Prelog's method was refined in 1956. The decarboxylation yield was increased by the addition of the Heinsdecker pathway (11%) and the Hoffman reaction (24%) that raised the total yield to 6.5%.[11][12] The process was still too complex, and a more convenient method was found in 1957 by Paul von Ragué Schleyer: dicyclopentadiene was first hydrogenated in the presence of a catalyst (e.g. platinum dioxide) and then transformed into adamantane using a Lewis acid (e.g. aluminium chloride) as another catalyst. This method increased the yield to 30–40% and provided an affordable source of adamantane; it therefore stimulated characterization of adamantane and is still used in laboratory practice.[13][14] The adamantane synthesis yield was later increased to 60%[15] and 98% by ultrasound and super acid catalysts. Today, adamantane is an affordable chemical compound with a cost of about $1 a gram.
All the above methods yield adamantane as a polycrystalline powder. Using this powder, single crystals can be grown from the melt, solution, or vapor phase (e.g. with the Bridgman–Stockbarger technique). Melt growth results in the worst crystalline quality with a mosaic spread in the X-ray reflection of about 1°. The best crystals are obtained from the liquid phase, but the growth is impracticably slow – several months for a 5–10 mm crystal. Growth from the vapor phase is a reasonable compromise in terms of speed and quality.[2] Adamantane is sublimed in a quartz tube placed in a furnace, which is equipped with several heaters maintaining a certain temperature gradient (about 10 °C/cm for adamantane) along the tube. Crystallization starts at one end of the tube, which is kept near the freezing point of adamantane. Slow cooling of the tube, while maintaining the temperature gradient, gradually shifts the melting zone (rate ~2 mm/hour) producing a single-crystal boule.[16]
Natural occurrence
Before adamantane was synthesized, it was isolated from petroleum by the Czech chemists S. Landa, V. Machacek and M. Mzourek in 1932[17] .[18] They used fractional distillation, which separates the organic molecule components of petroleum based on their boiling points. Landa et al. could produce only a few milligrams of adamantane, but noticed its high boiling and melting points. Because of the (assumed) similarity of its structure to that of diamond, the new compound was named adamantane.[8]
Petroleum remains the only natural source of adamantane; the content varies from between 0.0001% and 0.03% depending on the oil field and is too low for commercial production.[19][20]
Beside adamantane, petroleum contains more than thirty of its derivatives.[19] Their isolation from a complex mixture of hydrocarbons is possible due to their high melting point and the ability to distill with water vapor and form stable adducts with thiourea.
Physical properties
Pure adamantane is a colorless, crystalline solid with a characteristic camphor smell. It is practically insoluble in water, but readily soluble in nonpolar organic solvents.[21] Adamantane has an unusually high melting point for a hydrocarbon. At 270 °C, its melting point is much higher than other hydrocarbons with the same molecular weight, such as camphene (45 °C), limonene (−74 °C), ocimene (50 °C), terpinene (60 °C) or twistane (164 °C), or than a linear C10H22 hydrocarbon decane (−28 °C). However, adamantane slowly sublimes even at room temperature.[22] Adamantane can be distilled with water vapor.[20]
Structure
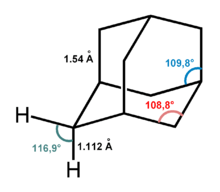
The adamantane molecule consists of three condensed cyclohexane rings fused in the armchair conformation. The molecular parameters were deduced by electron diffraction and X-ray crystallography. The carbon–carbon bond length is 1.54 Å, almost identical to that of diamond, and the carbon–hydrogen distance is 1.112 Å.[3]
At ambient conditions, adamantane crystallizes in a face-centered cubic structure (space group Fm3m, a = 9.426 ± 0.008 Å, four molecules in the unit cell) containing orientationally disordered adamantane molecules. This structure transforms into an ordered, primitive, tetragonal phase (a = 6.641 Å, c = 8.875 Å) with two molecules per cell, either upon cooling to 208 K or pressurizing to above 0.5 GPa.[8][22]
This phase transition is of the first order; it is accompanied by an anomaly in the heat capacity, elastic, and other properties. In particular, whereas adamantane molecules freely rotate in the cubic phase, they are frozen in the tetragonal one; the density increases stepwise from 1.08 to 1.18 g/cm3 and the entropy changes by a significant amount of 1594 J/(mol·K).[2]
Hardness
Elastic constants of adamantane were measured using large (centimeter-sized) single crystals and the ultrasonic echo technique. The principal value of the elasticity tensor, C11, was deduced as 7.52, 8.20, and 6.17 GPa for the <110>, <111>, and <100> crystalline directions.[16] For comparison, the corresponding values for crystalline diamond are 1161, 1174, and 1123 GPa.[23] The arrangement of carbon atoms is the same in adamantane and diamond.[24] However, in the adamantane solid, molecules do not form a covalent lattice as in diamond, but interact through weak Van der Waals forces. As a result, adamantane crystals are very soft and plastic.[2][16][25]
Spectroscopy
The nuclear magnetic resonance (NMR) spectrum of adamantane consists of two poorly resolved signals, which correspond to the inequivalent sites 1 and 2 (see picture below). Their positions are 1.873 ppm and 1.756 ppm for adamantane in CDCl3 and 1H NMR, and are 28.46 ppm and 37.85 ppm for 13C NMR.[26] The simplicity of the NMR spectrum is a good monitor of the purity of adamantane – most derivatives have lower molecular symmetry and therefore more complex spectra.
Mass spectra of adamantane and its derivatives are rather characteristic. The main peak at m/z = 136 corresponds to the C
10H+
16 ion. Its fragmentation results in weaker signals as m/z = 93, 80, 79, 67, 41 and 39.[3][26]
The infrared absorption spectrum of adamantane is relatively simple because of the high symmetry of the molecule. The main absorption bands and their assignment are given in the table:[3]
| Frequency of vibrations, cm−1 | Assignment* |
|---|---|
| 444 | δ(CCC) |
| 638 | δ(CCC) |
| 798 | ν(C−C) |
| 970 | ρ(CH2), ν(C−C), δ(HCC) |
| 1103 | δ(HCC) |
| 1312 | ν(C−C), ω(CH2) |
| 1356 | δ(HCC), ω(CH2) |
| 1458 | δ(HCH) |
| 2850 | ν(C−H) in CH2 groups |
| 2910 | ν(C−H) in CH2 groups |
| 2930 | ν(C−H) in CH2 groups |
* Legends correspond to different types of oscillations: δ – deformation, ν – stretching, ρ and ω – out of plane deformation vibrations of CH2 groups.
Optical activity
If adamantane molecules have four different substituents at every nodal carbon site, then they are chiral and optically active. As in biphenyls, the center of chirality does not belong to any particular carbon atom.[27] Such optical activity was described in adamantane in 1969 with the four different substituents being hydrogen, bromine and methyl and carboxyl group. The values of specific rotation are small and are usually within 1°.[28][29]
Nomenclature
Using the rules of systematic nomenclature, adamantane is called tricyclo[3.3.1.13,7]decane. However, IUPAC recommends using the name "adamantane".[1]
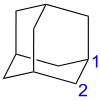
The adamantane molecule is composed of only carbon and hydrogen and has high Td symmetry. Therefore, its 16 hydrogen and 10 carbon atoms can be described by only two sites, which are labeled in the figure as 1 (4 equivalent sites) and 2 (6 equivalent sites).
The closest structural analogs of adamantane are noradamantane and homoadamantane, which respectively contain one less and one more CH2 link than the adamantane.
Chemical properties
Usually, hydrocarbons which contain only σ-bonds are relatively inert chemically. However, adamantane and its derivatives are highly reactive. This property is particularly evident in the ionic reactions where carbocations are formed as intermediates.
Adamantane cations
The adamantane cation can be produced by reacting 1-fluoro-adamantane with SbF5 and it has high stability compared with other carbocations, even tertiary ones.[30][31]
The dication of 1,3-didehydroadamantane was obtained in solutions of superacids. It also has elevated stability due to the phenomenon called "three-dimensional aromaticity"[32] or homoaromaticity.[33] This four-center two-electron bond involves one pair of electrons delocalized among the four bridgehead atoms.
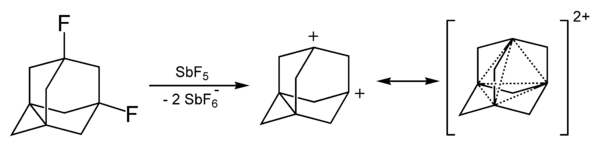
Reactions
Most reactions of adamantane occur via the 3-coordinated carbon sites and are described in the subsections below. The 2-coordinated, bridging carbon sites are much less reactive. They are involved in the reaction of adamantane with concentrated sulfuric acid which produces adamantanone.[34]
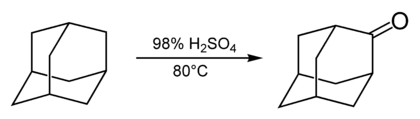
The carbonyl group of adamantanone allows further reactions via the bridging site. For example, adamantanone is the starting compound for obtaining such derivatives of adamantane as 2-adamantanecarbonitrile[35] and 2-methyl-adamantane.[36]
Bromination
Adamantane readily reacts with various brominating agents, including molecular bromine. The composition and the ratio of the reaction products depend on the reaction conditions and especially the presence and type of catalysts.[19]
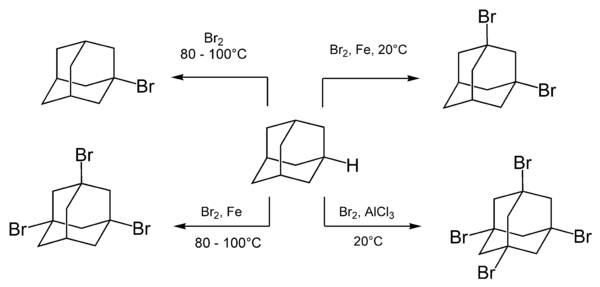
Boiling of adamantane with bromine results in a monosubstituted adamantane, 1-bromadamantane. Multiple substitution with bromine is achieved by adding a Lewis acid catalyst.[37]
The rate of bromination is accelerated upon addition of Lewis acids and is unchanged by irradiation or addition of free radicals. This indicates that the reaction occurs via an ionic mechanism.[8]
Fluorination
The first fluorinations of adamantane were conducted using 1-hydroxyadamantane[38] and 1-aminoadamantane as initial compounds. Later, fluorination was achieved starting from adamantane itself.[39] In all these cases, reaction proceeded via formation of adamantane cation which then interacted with fluorinated nucleophiles. Fluorination of adamantane with gaseous fluorine has also been reported.[40]
Carboxylation
Carboxylation of adamantane was first reported in 1960, using formic acid as a carboxylating agent and carbon tetrachloride as a solvent.[41]
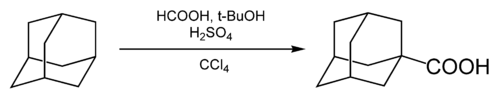
tert-butanol (t-BuOH) and sulfuric acid were added to generate adamantane cation; the cation was then carboxylated by carbon monoxide generated in situ in the interaction between the formic and sulfuric acids.[8] The fraction of carboxylated adamantane was 55-60%.[42]
Hydroxylation
The simplest adamantane alcohol, 1-hydroxyadamantane, is readily formed by hydrolysis of 1-bromadamantane in aqueous solution of acetone. It can also be produced by ozonation of the adamantane:[43]
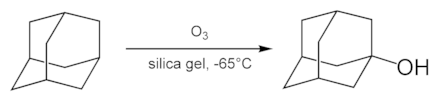
Others
Adamantane interacts with benzene in the presence of Lewis acids, resulting in a Friedel–Crafts reaction.[44] Aromatically substituted adamantane derivatives can be easily obtained starting from 1-hydroxyadamantane. In particular, the reaction with anisole proceeds under normal conditions and does not require a catalyst.[37]
Nitration of adamantane is a difficult reaction characterized by moderate yields.[45] An important nitrogen-substituted drug amantadine can be prepared by reacting adamantane with bromine or nitric acid to give the bromide or nitroester at the 1- position. Reaction of either compound with acetonitrile affords the acetamide, which is hydrolyzed to give 1-adamantylamine:[46]
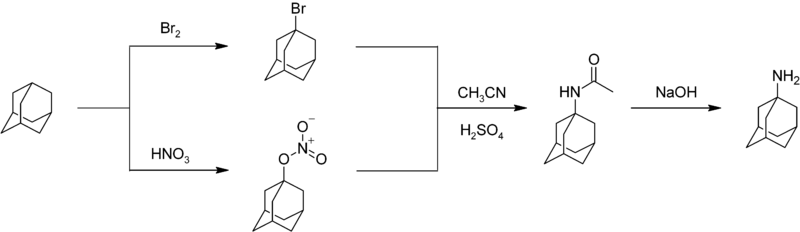
Uses
Adamantane itself enjoys few applications since it is merely an unfunctionalized hydrocarbon. It is used in some dry etching masks[47] and polymer formulations.
In solid-state NMR spectroscopy, adamantane is a common standard for chemical shift referencing.[48]
In dye lasers, adamantane may be used to extend the life of the gain medium; it cannot be photoionized under atmosphere because its absorption bands lie in the vacuum-ultraviolet region of the spectrum. Photoionization energies have been determined for adamantane as well as for several bigger diamondoids.[49]
In medicine
All medical applications known so far involve not pure adamantane, but its derivatives. The first adamantane derivative used as a drug was amantadine – first (1967) as an antiviral drug against various strains of flu[50] and then to treat Parkinson's disease.[51][52] Other drugs among adamantane derivatives include adapalene, adapromine, bromantane, carmantadine, chlodantane, dopamantine, memantine, rimantadine, saxagliptin, tromantadine, and vildagliptin. Polymers of adamantane have been patented as antiviral agents against HIV.[53]
Influenza virus strains have developed drug resistance to amantadine and rimantadine, which are not effective against prevalent strains as of 2016.
In designer drugs
Adamantane was recently identified as a key structural subunit in several synthetic cannabinoid designer drugs, namely AB-001 and SDB-001.[54]
Potential technological applications
Some alkyl derivatives of adamantane have been used as a working fluid in hydraulic systems.[55] Adamantane-based polymers might find application for coatings of touchscreens,[56] and there are prospects for using adamantane and its homologues in nanotechnology. For example, the soft cage-like structure of adamantane solid allow incorporation of guest molecules, which can be released inside the human body upon breaking the matrix.[15][57] Adamantane could be used as molecular building blocks for self-assembly of molecular crystals.[58][59]
Adamantane analogues
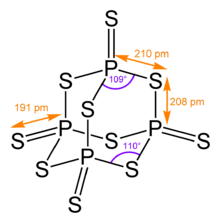
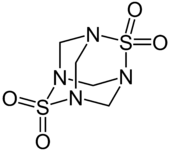
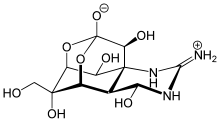
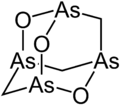
Many molecules adopt adamantane-like cage structures. Those include phosphorus trioxide P4O6, arsenic trioxide As4O6, phosphorus pentoxide P4O10 = (PO)4O6, phosphorus pentasulfide P4S10 = (PS)4S6, and hexamethylenetetramine C6N4H12 = N4(CH2)6.[60] Particularly notorious is tetramethylenedisulfotetramine, often shortened to "tetramine", a rodenticide banned in most countries for extreme toxicity to humans. The silicon analogue of adamantane, sila-adamantane, was synthesized in 2005.[61] Arsenicin A is a naturally occurring organoarsenic analogue isolated from the New Caledonian marine sponge Echinochalina bargibanti and is the first known polyarsenic organic compound.[62][63][64][65]
 Adamantane
Adamantane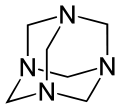

Adamantane cages can be stacked together to produce higher diamondoids, such as diamantane (C14H20 – two fused adamantane cages), triamantane (C18H24), tetramantane (C22H28), pentamantane (C26H32), hexamantane (C26H30), etc. Their synthesis is similar to that of adamantane and like adamantane, they can also be extracted from petroleum, though at even much smaller yields.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 169. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The retained names adamantane and cubane are used in general nomenclature and as preferred IUPAC names.
- Windsor, C G; Saunderson, D H; Sherwood, J N; Taylor, D; Pawley, G S (1978). "Lattice dynamics of adamantane in the disordered phase". Journal of Physics C: Solid State Physics. 11 (9): 1741–1759. Bibcode:1978JPhC...11.1741W. doi:10.1088/0022-3719/11/9/013.
- Bagrii, E.I. (1989). Adamantanes: synthesis, properties, applications (in Russian). Nauka. pp. 5–57. ISBN 5-02-001382-X.
- Alexander Senning. Elsevier's Dictionary of Chemoetymology. Elsevier, 2006, p. 6 ISBN 0-444-52239-5.
- Decker H. (1924). "Versammlung deutscher Naturforscher und Ärzte. Innsbruck, 21–27 September 1924". Angew. Chem. 37 (41): 795. doi:10.1002/ange.19240374102.
- Radcliffe, Marc D.; Gutierrez, Alberto; Blount, John F.; Mislow, Kurt (1984). "Structure of Meerwein's ester and of its benzene inclusion compound" (PDF). Journal of the American Chemical Society. 106 (3): 682–687. doi:10.1021/ja00315a037. Archived from the original (PDF) on 2011-08-09. Retrieved 2010-05-26.
- S. Coffey, S. Rodd (ed.) Chemistry of Carbon Compounds. Vol 2. Part C. Elsevier Publishing Co.: New York. 1969
- Fort, Raymond C. Jr.; Schleyers, Paul Von R. (1964). "Adamantane: Consequences of Diamondoid Structure". Chem. Rev. 64 (3): 277–300. doi:10.1021/cr60229a004.
- Prelog V, Seiwerth R (1941). "Über die Synthese des Adamantans". Berichte. 74 (10): 1644–1648. doi:10.1002/cber.19410741004.
- Prelog V, Seiwerth R (1941). "Über eine neue, ergiebigere Darstellung des Adamantans". Berichte. 74 (11): 1769–1772. doi:10.1002/cber.19410741109.
- Stetter, H., Bander, O., and Neumann, W., Ber., 89, 1922 (1956).
- McKervey, M (1980). "Synthetic approaches to large diamondoid hydrocarbons". Tetrahedron. 36 (8): 971–992. doi:10.1016/0040-4020(80)80050-0.
- Schleyer, P. von R. (1957). "A Simple Preparation of Adamantane". J. Am. Chem. Soc. 79 (12): 3292. doi:10.1021/ja01569a086.
- Schleyer, P. von R.; Donaldson, M. M.; Nicholas, R. D.; Cupas, C. (1973). "Adamantane". Organic Syntheses.; Collective Volume, 5, p. 16
- Mansoori, G. Ali (2007). Molecular building blocks for nanotechnology: from diamondoids to nanoscale materials and applications. Springer. pp. 48–55. ISBN 978-0-387-39937-9.
- Drabble, J R; Husain, A H M (1980). "Elastic properties of adamantane single crystals". Journal of Physics C: Solid State Physics. 13 (8): 1377–1380. Bibcode:1980JPhC...13.1377D. doi:10.1088/0022-3719/13/8/008.
- Landa, S.; Machácek, V. (1933). "Sur l'adamantane, nouvel hydrocarbure extrait de naphte". Collection of Czechoslovak Chemical Communications. 5: 1–5. doi:10.1135/cccc19330001.
- Landa, S.; Machacek, V.; Mzourek, M.; Landa, M. (1933), "Title unknown", Chim Ind. Spec. Publ. Vol. 506 (Abstracts of the 12th Conference of Industrial Chemistry, Prague, Sept. 1932); Chem. Abstr. 1933. Vol. 27. P. 5949.
- "Synthesis of adamantane" (in Russian). Retrieved 2009-12-11. Special practical problem for the students of IV year. Department of Petroleum Chemistry and Organic Catalysis MSU.
- Bagriy EI (1989). "Methods for hydrocarbon adamantane series". Adamantane: Synthesis, properties, application. Moscow: Nauka. pp. 58–123. ISBN 5-02-001382-X.
- "Adamantane". Encyclopedia of Chemistry (in Russian). Retrieved 2009-12-11.
- Vijayakumar, V.; et al. (2001). "Pressure induced phase transitions and equation of state of adamantane". J. Phys.: Condens. Matter. 13 (9): 1961–1972. Bibcode:2001JPCM...13.1961V. doi:10.1088/0953-8984/13/9/318.
- Anastassakis, E.; Siakavellas, M. (1999). "Elastic and Lattice Dynamical Properties of Textured Diamond Films". Physica Status Solidi B. 215 (1): 189–192. Bibcode:1999PSSBR.215..189A. doi:10.1002/(SICI)1521-3951(199909)215:1<189::AID-PSSB189>3.0.CO;2-X.
- Mansoori, G. Ali (2005). Principles of nanotechnology: molecular-based study of condensed matter in small systems. World Scientific. p. 12. ISBN 981-256-154-4.
- Wright, John Dalton (1995). Molecular crystals. Cambridge University Press. p. 28. ISBN 0-521-47730-1.
- NMR, IR and mass spectra of adamantane can be found in the SDBS database
- March, J. (1987). Organic chemistry. Reactions, mechanisms, structure. Advanced course for universities and higher education chemical. 1. M.: World. p. 137.
- Applequist, J.; Rivers, P.; Applequist, D. E. (1969). "Theoretical and experimental studies of optically active bridgehead-substituted adamantanes and related compounds". J. Am. Chem. Soc. 91 (21): 5705–5711. doi:10.1021/ja01049a002.
- Hamill, H.; McKervey, M. A. (1969). "The resolution of 3-methyl-5-bromoadamantanecarboxylic acid". Chem. Comm. (15): 864. doi:10.1039/C2969000864a.
- Schleyer P. R.; Fort R. C.; Watts W. E. (1964). "Stable Carbonium Ions. VIII. The 1-Adamantyl Cation". J. Am. Chem. Soc. 86 (19): 4195–4197. doi:10.1021/ja01073a058.
- Olah, George A.; Prakash, G. K. Surya; Shih, Joseph G.; Krishnamurthy, V. V.; Mateescu, Gheorge D.; Liang, Gao; Sipos, Gyorgy; Buss, Volker; Gund, Tamara M.; Schleyer, Paul v. R. (1985). "Bridgehead adamantyl, diamantyl, and related cations and dications". J. Am. Chem. Soc. 107 (9): 2764–2772. doi:10.1021/ja00295a032.
- Smith, W.; Bochkov A.; Caple, R. (2001). Organic Synthesis. Science and art. M.: World. p. 573. ISBN 5-03-003380-7.
- Bremer, Matthias; von Ragué Schleyer, Paul; Schötz, Karl; Kausch, Michael; Schindler, Michael (1987). "Four-Center Two-Electron Bonding in a Tetrahedral Topology. Experimental Realization of Three-Dimensional Homoaromaticity in the 1,3-Dehydro-5,7-adamantanediyl Dication". Angewandte Chemie International Edition in English. 26 (8): 761–763. doi:10.1002/anie.198707611.
- Geluk, H. W. and Keizer, V. G. "Adamantanone" Organic Syntheses, Coll. Vol. 6, p. 48 (1988); Vol. 53, p. 8 (1973) doi:10.15227/orgsyn.053.0008.
- 2-Adamantanecarbonitrile Archived 2012-07-10 at the Wayback Machine Organic Syntheses, Coll. Vol. 6, p. 41 (1988); Vol. 57, p. 8 (1977).
- Schleyer P. R.; Nicholas R. D. (1961). "The Preparation and Reactivity of 2-Substituted Derivatives of Adamantane". J. Am. Chem. Soc. 83 (1): 182–187. doi:10.1021/ja01462a036.
- Nesmeyanov, A. N. (1969). Basic organic chemistry (in Russian). p. 664.
- Olah, George A.; Welch, John T.; Vankar, Yashwant D.; Nojima, Mosatomo; Kerekes, Istvan; Olah, Judith A. (1979). "Pyridinium poly (hydrogen fluoride): a convenient reagent for organic fluorination reactions". Journal of Organic Chemistry. 44 (22): 3872–3881. doi:10.1021/jo01336a027.
- Olah, George A.; Shih, Joseph G.; Singh, Brij P.; Gupta, B. G. B. (1983). "Ionic fluorination of adamantane, diamantane, and triphenylmethane with nitrosyl tetrafluoroborate/pyridine polyhydrogen fluoride (PPHF)". Journal of Organic Chemistry. 48 (19): 3356–3358. doi:10.1021/jo00167a050.
- Rozen, Shlomo.; Gal, Chava (1988). "Direct synthesis of fluoro-bicyclic compounds with fluorine". Journal of Organic Chemistry. 53 (12): 2803–2807. doi:10.1021/jo00247a026.
- Koch, H.; Haaf, W. (1960). "Direkte Synthese der Adamantan-carbonsäure-(1)". Angewandte Chemie. 72 (17): 628. doi:10.1002/ange.19600721710.
- 1-Adamantanecarboxylic acid Organic Syntheses, Coll. Vol. 5, p. 20 (1973); Vol. 44, p. 1 (1964).
- Zvi Cohen, Haim Varkony, Ehud Keinan, and Yehuda Mazur Tertiary alcohols from hydrocarbons by ozonation on silica gel: 1-adamantanol Organic Syntheses, Coll. Vol. 6, p. 43 (1988); Vol. 59, p. 176 (1979)
- Chalais, Stephane; Corn lis, Andr; Gerstmans, Andr; Ko?odziejski, Wac?aw; Laszlo, Pierre; Mathy, Arthur; M tra, Pierre (1985). "Direct clay-catalyzed Friedel-Crafts arylation and chlorination of the hydrocarbon adamantane". Helvetica Chimica Acta. 68 (5): 1196–1203. doi:10.1002/hlca.19850680516.
- Smith, George W.; Williams, Harry D. (1961). "Some Reactions of Adamantane and Adamantane Derivatives". J. Org. Chem. 26 (7): 2207–2212. doi:10.1021/jo01351a011.
- Moiseev, I. K.; Doroshenko, R. I.; Ivanova, V. I. (1976). "Synthesis of amantadine via the nitrate of 1-adamantanol". Pharmaceutical Chemistry Journal. 10 (4): 450–451. doi:10.1007/BF00757832.
- Watanabe, Keiji; et al. (2001). "Resist Composition and Pattern Forming Process". United States Patent Application 20010006752. Bandwidth Market, Ltd. Archived from the original on September 4, 2011. Retrieved 14 October 2005.
- Morcombe, Corey R.; Zilm, Kurt W. (2003). "Chemical Shift referencing in MAS solid state NMR". J. Magn. Reson. 162 (2): 479–486. Bibcode:2003JMagR.162..479M. doi:10.1016/S1090-7807(03)00082-X. PMID 12810033.
- Lenzke, K.; Landt, L.; Hoener, M.; et al. (2007). "Experimental determination of the ionization potentials of the first five members of the nanodiamond series". J. Chem. Phys. 127 (8): 084320. Bibcode:2007JChPh.127h4320L. doi:10.1063/1.2773725. PMID 17764261. S2CID 3131583.
- Maugh, T. (1979). "Panel urges wide use of antiviral drug". Science. 206 (4422): 1058–60. Bibcode:1979Sci...206.1058M. doi:10.1126/science.386515. PMID 386515.
- Sonnberg, Lynn (2003). The Complete Pill Guide: Everything You Need to Know about Generic and Brand-Name Prescription Drugs. Barnes & Noble Publishing. p. 87. ISBN 0-7607-4208-1.
- Blanpied TA, Clarke RJ, Johnson JW (2005). "Amantadine inhibits NMDA receptors by accelerating channel closure during channel block". Journal of Neuroscience. 25 (13): 3312–22. doi:10.1523/JNEUROSCI.4262-04.2005. PMC 6724906. PMID 15800186.
- Boukrinskaia, A. G.; et al. "Polymeric Adamantane Analogues" (U.S. Patent 5,880,154). Retrieved 2009-11-05.
- Banister, S. D.; Wilkinson, S. M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2013). "The synthesis and pharmacological evaluation of adamantane-derived indoles: Novel cannabimimetic drugs of abuse". ACS Chemical Neuroscience. 4 (7): 1081–92. doi:10.1021/cn400035r. PMC 3715837. PMID 23551277.
- "Adamantane". Krugosvet (in Russian). Archived from the original on 6 November 2009. Retrieved 2009-11-11.
- Jeong, H. Y. (2002). "Synthesis and characterization of the first adamantane-based poly (p-phenylenevinylene) derivative: an intelligent plastic for smart electronic displays". Thin Solid Films. 417 (1–2): 171–174. Bibcode:2002TSF...417..171J. doi:10.1016/S0040-6090(02)00569-2.
- Ramezani, Hamid; Mansoori, G. Ali (2007). Diamondoids as Molecular Building Blocks for Nanotechnology. Topics in Applied Physics. 109. pp. 44–71. doi:10.1007/978-0-387-39938-6_4. ISBN 978-0-387-39937-9.
- Markle, R. C. (2000). "Molecular building blocks and development strategies for molecular nanotechnology". Nanotechnology. 11 (2): 89–99. Bibcode:2000Nanot..11...89M. doi:10.1088/0957-4484/11/2/309.
- Garcia, J. C.; Justo, J. F.; Machado, W. V. M.; Assali, L. V. C. (2009). "Functionalized adamantane: building blocks for nanostructure self-assembly". Phys. Rev. B. 80 (12): 125421. arXiv:1204.2884. Bibcode:2009PhRvB..80l5421G. doi:10.1103/PhysRevB.80.125421.
- Vitall, J. J. (1996). "The Chemistry of Inorganic and Organometallic Compounds with Adamantane-Like Structures". Polyhedron. 15 (10): 1585–1642. doi:10.1016/0277-5387(95)00340-1.
- Fischer, Jelena; Baumgartner, Judith; Marschner, Christoph (2005). "Synthesis and Structure of Sila-Adamantane". Science. 310 (5749): 825. doi:10.1126/science.1118981. PMID 16272116.
- Mancini, Ines; Guella, Graziano; Frostin, Maryvonne; Hnawia, Edouard; Laurent, Dominique; Debitus, Cecile; Pietra, Francesco (2006). "On the First Polyarsenic Organic Compound from Nature: Arsenicin a from the New Caledonian Marine Sponge Echinochalina bargibanti". Chemistry: A European Journal. 12 (35): 8989–94. doi:10.1002/chem.200600783. PMID 17039560.
- Tähtinen, Petri; Saielli, Giacomo; Guella, Graziano; Mancini, Ines; Bagno, Alessandro (2008). "Computational NMR Spectroscopy of Organoarsenicals and the Natural Polyarsenic Compound Arsenicin A". Chemistry: A European Journal. 14 (33): 10445–52. doi:10.1002/chem.200801272. PMID 18846604.
- Guella, Graziano; Mancini, Ines; Mariotto, Gino; Rossi, Barbara; Viliani, Gabriele (2009). "Vibrational analysis as a powerful tool in structure elucidation of polyarsenicals: a DFT-based investigation of arsenicin A". Physical Chemistry Chemical Physics. 11 (14): 2420–2427. Bibcode:2009PCCP...11.2420G. doi:10.1039/b816729j. PMID 19325974.
- Di Lu; A. David Rae; Geoff Salem; Michelle L. Weir; Anthony C. Willis; S. Bruce Wild (2010). "Arsenicin A, A Natural Polyarsenical: Synthesis and Crystal Structure". Organometallics. 29 (1): 32–33. doi:10.1021/om900998q.