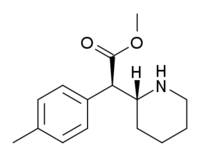
4-Methylmethylphenidate
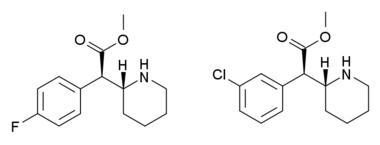
threo-4-Methylmethylphenidate (4-MeTMP) is a stimulant drug related to methylphenidate. It is slightly less potent than methylphenidate and has relatively low efficacy at blocking dopamine reuptake despite its high binding affinity, which led to its investigation as a possible substitute drug for treatment of stimulant abuse (cf. nocaine).[1] On the other hand, several other simple ring-substituted derivatives of threo-methylphenidate such as the 4-fluoro and 3-chloro compounds are more potent than methylphenidate both in efficacy as dopamine reuptake inhibitors and in animal drug discrimination assays.[2][3][4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Legality
4-Methylmethylphenidate was banned in the UK as a Temporary Class Drug from June 2015 following its unapproved sale as a designer drug.[5][6][7]
See also
References
- Wayment HK, Deutsch H, Schweri MM, Schenk JO (March 1999). "Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists?". Journal of Neurochemistry. 72 (3): 1266–74. doi:10.1046/j.1471-4159.1999.0721266.x. PMID 10037500.
- Deutsch HM, Shi Q, Gruszecka-Kowalik E, Schweri MM (March 1996). "Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs". Journal of Medicinal Chemistry. 39 (6): 1201–9. doi:10.1021/jm950697c. PMID 8632426.
- Schweri MM, Deutsch HM, Massey AT, Holtzman SG (May 2002). "Biochemical and behavioral characterization of novel methylphenidate analogs". The Journal of Pharmacology and Experimental Therapeutics. 301 (2): 527–35. doi:10.1124/jpet.301.2.527. PMID 11961053.
- Davies HM, Hopper DW, Hansen T, Liu Q, Childers SR (April 2004). "Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites". Bioorganic & Medicinal Chemistry Letters. 14 (7): 1799–802. doi:10.1016/j.bmcl.2003.12.097. PMID 15026075.
- Methylphenidate-based NPS: A review of the evidence of use and harm. Advisory Council on the Misuse of Drugs, 31 March 2015
- "Letter to Mike Penning on methylphenidate-based novel psychoactive substances". Advisory Council on the Misuse of Drugs. 25 June 2015. Retrieved 25 June 2015.
- "Ministerial response to the Advisory Council on the Misuse of Drugs about 2 new methylphenidate-based substances". Home Office. 25 June 2015. Retrieved 25 June 2015.