alpha-Pyrrolidinohexiophenone
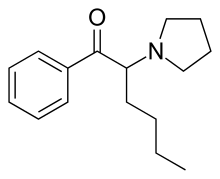
α-Pyrrolidinohexiophenone (α-PHP, alpha-PHP, α-Pyrrolidinohexanophenone, PV-7) is a synthetic stimulant drug of the cathinone class developed in the 1960s[1] which has been reported as a novel designer drug.[2][3][4][5][6]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intranasal, vaporization, intravenous, rectal, sublingual, subcutaneous |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H23NO |
| Molar mass | 245.366 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Similar chemical compounds
α-Pyrrolidinohexiophenone is a longer chain homologue of α-PVP, having an extra carbon on the alkyl side chain. Regarding the potency of alpha-PHP in the brain, chemist Michael H. Baumann of the Designer Drug Research Unit (established by Baumann[7]) of the National Institute on Drug Abuse stated: "alpha-PHP might be even more potent than alpha-PVP"; this statement is based on laboratory tests of chemical reactivity.[8]
Pyrovalerone is a structural isomer of alpha-PHP.[9]
Legality
In the United States, α-PHP is a Schedule I Controlled Substance.[10]
The President of the Republic of Italy classified cathinone and all structurally derived analogues (including pyrovalerone analogues) as narcotics on January 2012.[11][12]
Sweden's public health agency suggested to classify α-PHP as narcotic on June 1, 2015.[13]
As of October 2015, α-PHP is a controlled substance in China.[14]
In December 2019, the UNODC announced scheduling recommendations placing Alpha-PHP into Schedule II.[15]
References
- "US Patent 3314970 - α-Pyrrolidino ketones". Ernst Seeger. Retrieved 18 June 2015.
- Nahoko Uchiyama; Yoshihiko Shimokawa; Maiko Kawamura; Ruri Kikura-Hanajiri; Takashi Hakamatsuka (August 2014). "Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products". Forensic Toxicology. 32 (2): 266–281. doi:10.1007/s11419-014-0238-5.
- Milena Majchrzak; Marcin Rojkiewicz; Rafał Celiński; Piotr Kuś; Mieczysław Sajewicz (October 2015). "Identification and characterization of new designer drug 4-fluoro-PV9 and α-PHP in the seized materials". Forensic Toxicology. 34: 115–124. doi:10.1007/s11419-015-0295-4. PMC 4705138. PMID 26793278.
- Michael Paul; Sergej Bleicher; Susanne Guber; Josef Ippisch; Aldo Polettini; Wolfgang Schultis (November 2015). "Identification of phase I and II metabolites of the new designer drug α-pyrrolidinohexiophenone (α-PHP) in human urine by liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS)". Journal of Mass Spectrometry. 50 (11): 1305–1317. doi:10.1002/jms.3642. PMID 26505776.
- "α-Pyrrolidinohexanophenone". Cayman Chemical. Retrieved 17 June 2015.
- Janez Klavž; Maksimiljan Gorenjak; Martin Marinšek (August 2016). "Suicide attempt with a mix of synthetic cannabinoids and synthetic cathinones: Case report of non-fatal intoxication with AB-CHMINACA, AB-FUBINACA, alpha-PHP, alpha-PVP and 4-CMC". Forensic Science International. 265: 121–124. doi:10.1016/j.forsciint.2016.01.018. PMID 26890319.
- https://irp.drugabuse.gov/staff-members/michael-baumann/
- https://nymag.com/intelligencer/2016/09/the-obscure-legal-drug-that-fuels-john-mcafee.html - author: Jeff Wise
- Meltzer PC, Butler D, Deschamps JR, Madras BK (February 2006). "1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogs. A promising class of monoamine uptake inhibitors". Journal of Medicinal Chemistry. 49 (4): 1420–1432. doi:10.1021/jm050797a. PMC 2602954. PMID 16480278.
- "Schedules of Controlled Substances: Temporary Placement of N-Ethylhexedrone, α-PHP, 4-MEAP, MPHP, PV8, and 4-Chloro-α-PVP in Schedule I". Drug Enforcement Administration.
- "Decreto 29 dicembre 2011 (12A00013) (G.U. Serie Generale n. 3 del 4 gennaio 2012)" (PDF). Archived from the original (PDF) on 2016-07-01.
- "EMCDDA–Europol Joint Report on a new psychoactive substance: 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (α-PVP)". European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). September 2015.
- "23 nya ämnen kan klassas som narkotika eller hälsofarlig vara". Retrieved 29 June 2015.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 2015-10-01.
- "December 2019 – WHO: World Health Organization recommends 12 NPS for scheduling".