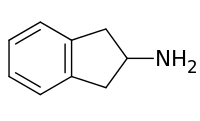
2-Aminoindane
2-Aminoindane (2-AI) is a research chemical with applications in neurologic disorders and psychotherapy that has also been sold as a designer drug.[1] It acts as a selective substrate for NET and DAT.[2][3]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2-indanylamine; 2-indanamine |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.111 |
| Chemical and physical data | |
| Formula | C9H11N |
| Molar mass | 133.194 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Therapeutic and illicit uses
Synthetic aminoindanes were originally developed in the context of anti-Parkinsonian drugs as a metabolite of rasagiline and as a tool to be used in psychotherapy. Deaths related to their toxic effects have been observed both in the laboratory in animal studies and in clinical encounters.[4] 2-AI is a rigid analogue of amphetamine and partially substitutes for it in rat discrimination tests.[5]
Legal status
Sweden's public health agency suggested classifying 2-AI as a hazardous substance, on June 24, 2019.[6]
Chemical derivatives
There are a number of derivatives of 2-aminoindane and its positional isomer 1-aminoindane exist, including:
Legality
China
As of October 2015 2-AI is a controlled substance in China.[7]
United States
2-Aminoindane is not scheduled at the federal level in the United States,[8] but may be considered an analog of amphetamine, in which case purchase, sale, or possession could be prosecuted under the Federal Analog Act.
See also
- 2-Aminodilin (2-AD)
- 2-Aminotetralin (2-AT)
- Amphetamine
- Bath salts (drug)
References
- Manier SK, Felske C, Eckstein N, Meyer MR (October 2019). "The metabolic fate of two new psychoactive substances - 2-aminoindane and N-methyl-2-aminoindane - studied in vitro and in vivo to support drug testing". Drug Testing and Analysis. doi:10.1002/dta.2699. PMID 31667988.
- Halberstadt AL, Brandt SD, Walther D, Baumann MH (March 2019). "2-adrenergic receptors". Psychopharmacology. 236 (3): 989–999. doi:10.1007/s00213-019-05207-1. PMC 6848746. PMID 30904940.
- Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME (March 2014). "Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives" (PDF). Biochemical Pharmacology. 88 (2): 237–44. doi:10.1016/j.bcp.2014.01.024. PMID 24486525.
- Pinterova N, Horsley RR, Palenicek T (2017). "Synthetic Aminoindanes: A Summary of Existing Knowledge". Frontiers in Psychiatry. 8: 236. doi:10.3389/fpsyt.2017.00236. PMC 5698283. PMID 29204127.
- Oberlender R, Nichols DE (March 1991). "Structural variation and (+)-amphetamine-like discriminative stimulus properties". Pharmacology, Biochemistry, and Behavior. 38 (3): 581–6. doi:10.1016/0091-3057(91)90017-V. PMID 2068194.
- "Åtta ämnen föreslås klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 24 June 2019.
- "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- 21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.