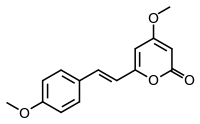
Yangonin
Yangonin is one of the six major kavalactones found in the kava plant.[1] It has been shown to possess binding affinity for the cannabinoid receptor CB1 (Ki = 0.72 μM), and selectivity vs. the CB₂ receptor (K(i)>10 μM) where it behaves as an agonist. The CB₁ receptor affinity of yangonin suggests that the endocannabinoid system might contribute to the complex human psychopharmacology of the traditional kava drink and the anxiolytic preparations obtained from the kava plant.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-Methoxy-6-[(E)-2-(4-methoxyphenyl)ethenyl]pyran-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.211.821 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H14O4 | |
| Molar mass | 258.273 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Toxicity
Yangonin displays marked in vitro toxicity on human hepatocytes with approximately 40% reduction in viability based on an ethidium bromide assay. The predominant mode of cell death turned out to be apoptosis rather than necrosis. No significant changes were observed in glutathione levels.[3]
References
- Malani, Joji (2002-12-03). "Evaluation of the effects of Kava on the Liver" (PDF). Fiji School of Medicine. Retrieved 2009-09-04.
- Ligresti, A.; Villano, R.; Allarà, M.; Ujváry, I. N.; Di Marzo, V. (2012). "Kavalactones and the endocannabinoid system: The plant-derived yangonin is a novel CB1 receptor ligand". Pharmacological Research. 66 (2): 163–169. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- Tang, J; Dunlop, RA; Rowe, A; Rodgers, KJ; Ramzan, I (2010). "Kavalactones Yangonin and Methysticin Induce Apoptosis in Human Hepatocytes (HepG2) In Vitro". Phytotherapy Research. 25 (3): 417–23. doi:10.1002/ptr.3283. PMID 20734326.