Mexazolam
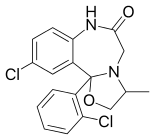
Mexazolam[1] (marketed under the trade names Melex and Sedoxil)[2] is a drug which is a benzodiazepine derivative.[3] Mexazolam has been trialed for anxiety and was found to be effective in alleviating anxiety at one week follow-up, however, after three weeks of therapy mexazolam had lost its therapeutic anxiolytic properties becoming no more effective than placebo, presumably due to benzodiazepine tolerance.[4] Mexazolam is metabolised via the CYP3A4 pathway. HMG-CoA reductase inhibitors including simvastatin, simvastatin acid, lovastatin, fluvastatin, atorvastatin and cerivastatin inhibit the metabolism of mexazolam,[5] but not the HMG-CoA reductase inhibitor pravastatin.[6][7] Its principal active metabolites are chlordesmethyldiazepam (also known as chloronordiazepam or delorazepam, trade name Dadumir) and chloroxazepam (also known as lorazepam, trade name Ativan).[8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Melex, Sedoxil |
| Other names | 13-chloro- 2-(2-chlorophenyl)- 5-methyl- 3-oxa- 6,9-diazatricyclo[8.4.0.02,6] tetradeca- 1(10),11,13-trien- 8-one |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP3A4) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H16Cl2N2O2 |
| Molar mass | 363.24 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
See also
References
- DE Patent 1954065
- "Benzodiazepine Names". non-benzodiazepines.org.uk. Archived from the original on 2008-12-08. Retrieved 2009-04-05.
- Kurono Y, Kamiya K, Kuwayama T, Jinno Y, Yashiro T, Ikeda K (September 1987). "Kinetics and mechanism of the acid-base equilibrium of mexazolam and comparison with those of other commercial benzodiazepinooxazole drugs". Chemical & Pharmaceutical Bulletin. 35 (9): 3831–7. doi:10.1248/cpb.35.3831. PMID 2893667.
- Ferreira L, Figueira ML, Bessa-Peixoto A, Marieiro A, Albuquerque R, Paz C, et al. (2003). "Psychomotor and anxiolytic effects of mexazolam in patients with generalised anxiety disorder". Clinical Drug Investigation. 23 (4): 235–43. doi:10.2165/00044011-200323040-00003. PMID 17535036.
- Mc Donnell CG, Harte S, O'Driscoll J, O'Loughlin C, Van Pelt FN, Shorten GD, Van Pelt FD (September 2003). "The effects of concurrent atorvastatin therapy on the pharmacokinetics of intravenous midazolam". Anaesthesia. 58 (9): 899–904. doi:10.1046/j.1365-2044.2003.03339.x. PMID 12911366.
- Ishigami M, Takasaki W, Ikeda T, Komai T, Ito K, Sugiyama Y (August 2002). "Sex difference in inhibition of in vitro mexazolam metabolism by various 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors in rat liver microsomes". Drug Metabolism and Disposition. 30 (8): 904–10. doi:10.1124/dmd.30.8.904. PMID 12124308.
- Ishigami M, Honda T, Takasaki W, Ikeda T, Komai T, Ito K, Sugiyama Y (March 2001). "A comparison of the effects of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase inhibitors on the CYP3A4-dependent oxidation of mexazolam in vitro". Drug Metabolism and Disposition. 29 (3): 282–8. PMID 11181496.
- Helder Fernandes; Ricardo Moreira (2014). "Mexazolam: Clinical Efficacy and Tolerability in the Treatment of Anxiety". Neurology and Therapy. 3 (1): 1–14. doi:10.1007/s40120-014-0016-7. PMC 4381915. PMID 26000220.