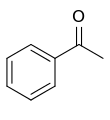
Acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3 (also represented by the pseudoelement symbols PhAc or BzMe). It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Phenylethan-1-one[1] | |||
| Other names
Acetophenone Phenylethanone Phenylacetone Methyl phenyl ketone | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | ACP | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.462 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H8O | |||
| Molar mass | 120.151 g·mol−1 | ||
| Density | 1.028 g/cm3 | ||
| Melting point | 19–20 °C (66–68 °F; 292–293 K) | ||
| Boiling point | 202 °C (396 °F; 475 K) | ||
| 5.5 g/L at 25 °C 12.2 g/L at 80 °C | |||
| -72.05·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | MSDS | ||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H302, H319 | ||
| P264, P270, P280, P301+312, P305+351+338, P330, P337+313, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77 °C (171 °F; 350 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
Acetophenone is recovered as a by-product of the oxidation of ethylbenzene to ethylbenzene hydroperoxide. Ethylbenzene hydroperoxide is an intermediate in the commercial production of propylene oxide via the propylene oxide - styrene co-product process.[3] Ethylbenzene hydroperoxide is primarily converted to 1-phenylethanol (α-methylbenzyl alcohol) in the process with a small amount of by-product acetophenone. Acetophenone is recovered or hydrogenated to 1-phenylethanol which is then dehydrated to produce styrene.[2]
Uses
Precursor to resins
Commercially significant resins are produced from treatment of acetophenone with formaldehyde and a base. The resulting copolymers are conventionally described with the formula [(C6H5COCH)x(CH2)x]n, resulting from aldol condensation. These substances are components of coatings and inks. Modified acetophenone-formaldehyde resins are produced by the hydrogenation of the aforementioned ketone-containing resins. The resulting polyol can be further crosslinked with diisocyanates.[2] The modified resins are found in coatings, inks and adhesives.
Niche uses
Acetophenone is an ingredient in fragrances that resemble almond, cherry, honeysuckle, jasmine, and strawberry. It is used in chewing gum.[4] It is also listed as an approved excipient by the U.S. FDA.[5]
Laboratory reagent
In instructional laboratories,[6] acetophenone is converted to styrene in a two-step process that illustrates the reduction of carbonyls using hydride and the dehydration of alcohols:
- 4 C6H5C(O)CH3 + NaBH4 + 4 H2O → 4 C6H5CH(OH)CH3 + NaOH + B(OH)3 → C6H5CH=CH2
A similar two-step process is used industrially, but reduction step is performed by hydrogenation over a copper catalyst.[2]
- C6H5C(O)CH3 + H2 → C6H5CH(OH)CH3
Being prochiral, acetophenone is also a popular test substrate for asymmetric hydrogenation experiments.
Drugs
Acetophenone is used for the synthesis of many pharmaceuticals.[7][8]
A Mannich reaction with dimethylamine and formaldehyde gives β-dimethylaminopropiophenone.[9] Using diethylamine instead gives the diethylamino analog.
Natural occurrence
Acetophenone occurs naturally in many foods including apple, cheese, apricot, banana, beef, and cauliflower. It is also a component of castoreum, the exudate from the castor sacs of the mature beaver.[10]
Pharmacology
In the late 19th and early 20th centuries, acetophenone was used in medicine.[11] It was marketed as a hypnotic and anticonvulsant under brand name Hypnone. The typical dosage was 0.12 to 0.3 milliliters.[12] It was considered to have superior sedative effects to both paraldehyde and chloral hydrate.[13] In humans, acetophenone is metabolized to benzoic acid, carbonic acid, and acetone.[14] Hippuric acid occurs as an indirect metabolite and its quantity in urine may be used to confirm acetophenone exposure,[15] although other substances, like toluene, also induce hippuric acid in urine.[16]
Toxicity
The LD50 is 815 mg/kg (oral, rats).[2] Acetophenone is currently listed as a Group D carcinogen indicating that it does not produce carcinogenic effects in humans.
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 723. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
The names acetophenone and benzophenone are retained only for general nomenclature, but no substitution is allowed.
- Siegel, Hardo; Eggersdorfer, Manfred. "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.
- "Archived copy" (PDF). Archived from the original (PDF) on 2017-10-07. Retrieved 2017-10-07.CS1 maint: archived copy as title (link)
- Burdock, George A. (2005), Fenaroli's Handbook of Flavor Ingredients (5th ed.), CRC Press, p. 15, ISBN 0-8493-3034-3, archived from the original on 2014-09-25
- "Inactive Ingredient Search for Approved Drug Products". Archived from the original on 2013-05-04.
- Wilen, Samuel H.; Kremer, Chester B.; Waltcher, Irving (1961). "Polystyrene—A multistep synthesis: For the undergraduate organic chemistry laboratory". J. Chem. Educ. 38 (6): 304–305. Bibcode:1961JChEd..38..304W. doi:10.1021/ed038p304.
- Sittig, Marshall (1988). Pharmaceutical Manufacturing Encyclopedia. pp. 39, 177. ISBN 978-0-8155-1144-1.
- Gadamasetti, Kumar; Tamim Braish (2007). Process Chemistry in the Pharmaceutical Industry, Volume 2. pp. 142–145. ISBN 978-0-8493-9051-7.
- Maxwell, Charles E. (1943). "β-Dimethylaminopropiophenone Hydrochloride". Organic Syntheses. 23: 30.
- Müller-Schwarze, D.; Houlihan, P. W. (April 1991). "Pheromonal activity of single castoreum constituents in beaver, Castor canadensis". Journal of Chemical Ecology. 17 (4): 715–34. doi:10.1007/BF00994195. PMID 24258917.
- Budavari, Susan, ed. (1996), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123
- Bartholow, Roberts (1908). A Practical Treatise on Materia Medica and Therapeutics. Appleton & Co.
- Norman, Conolly (1887). "Cases illustrating the sedative effects of aceto-phenone". Journal of Mental Science. 32: 519. doi:10.1192/bjp.32.140.519.
- "Hypnone – The new hypnotic". Journal of the American Medical Association. 5 (23): 632. 1885. doi:10.1001/jama.1885.02391220016006.
- CID 7410 from PubChem
- "The Netherlands Center for Occupational Diseases (NCvB): toluene (Dutch)" (PDF). beroepsziekten.nl. Retrieved 19 April 2018.