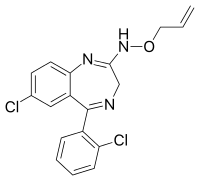
Uldazepam
Uldazepam is a drug which is a benzodiazepine derivative.[1] It has sedative and anxiolytic effects similar to those of other benzodiazepines.[2][3]
 | |
| Clinical data | |
|---|---|
| Other names | 7-chloro-5-(2-chlorophenyl)-N-prop-2-enoxy-3H-1,4-benzodiazepin-2-amine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H15Cl2N3O |
| Molar mass | 360.24 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
Thio thionamide is even more prone to amidine formation than the lactam itself.
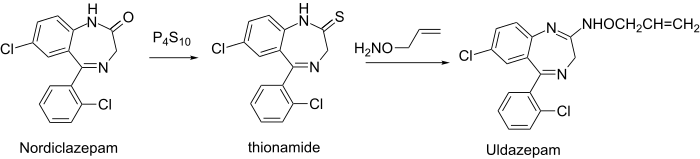
Uldazepam synthesis: J. B. Hester, Jr., DE 2005176 (1970); Chem. Abstr., 73: 99,001t (1970).
Reaction of thionamide (2) with O-allyl-hydroxylamine gave the oximino (3) uldazepam.
gollark: Again, people are inconsistent and weird.
gollark: Of course!
gollark: Especially on the https://dragcave.net/view/n/Trade%20Hub%20Feedback%20Thread
gollark: People: Inconsistent and Weird.
gollark: (as I said on the thread)
See also
References
- "Uldazepam U 31920". Psychotropics. Retrieved 12 June 2013.
- Oelschläger H, Ellaithy MM, Volke J. Mechanism of the polarographic reduction of the tranquilizer uldazepam. (German). Archiv der Pharmazie (Weinheim). 1988 Feb;321(2):69-72.
- Itil TM, Akpinar S, Ozkut H, et al.: Clinical and computerized EEG effects of U-31,920 a new anxiolytic. Current Therapeutic Research. 1974; 16: 642-654.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.