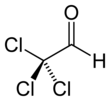
Chloral
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloroacetaldehyde | |||
| Other names
Trichloroethanal | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 506422 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.829 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2HCl3O | |||
| Molar mass | 147.38 g·mol−1 | ||
| Appearance | Colorless, mobile, oily liquid | ||
| Odor | Pungent and irritating | ||
| Density | 1.404 g/cm3 | ||
| Melting point | −57.5 °C (−71.5 °F; 215.7 K) | ||
| Boiling point | 97.8 °C (208.0 °F; 370.9 K) | ||
| Forms soluble hydrate | |||
| Solubility in ethanol | Miscible | ||
| Solubility in diethyl ether | Miscible | ||
| Solubility in chloroform | Miscible | ||
| Acidity (pKa) | 9.66 | ||
| −6.77×10−5 cm3/mol | |||
Refractive index (nD) |
9.48846 | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H301, H302, H315, H319, H335 | ||
| P261, P264, P270, P271, P280, P301+310, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
480 mg/kg (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
Chloral was first prepared, and named, by the German chemist Justus von Liebig in 1832.[1] Liebig treated anhydrous ethanol with dry chlorine gas.[2]
Chloral is produced commercially by the chlorination of acetaldehyde in the presence of hydrochloric acid, producing chloral hydrate. Ethanol can also be used as a feedstock. This reaction is catalyzed by antimony trichloride:
- H3CCHO + 3 Cl2 + H2O → Cl3CCH(OH)2 + 3 HCl
The chloral hydrate is distilled from the reaction mixture. The distillate is then dehydrated with concentrated sulfuric acid, after which the heavier acid layer (containing the water) is drawn off:
- Cl3CCH(OH)2 → Cl3CCHO + H2O
The resulting product is purified by fractional distillation.[3] Small amounts of chloral hydrate occur in some chlorinated water.
Key reactions
Chloral tends to form adducts with water (to give chloral hydrate) and alcohols.
Aside from its tendency to hydrate, chloral is notable as a building block in the synthesis of DDT. For this purpose, chloral is treated with chlorobenzene in the presence of a catalytic amount of sulfuric acid:
- Cl3CCHO + 2 C6H5Cl → Cl3CCH(C6H4Cl)2 + H2O
This reaction was described by Othmar Zeidler in 1874.[4] The related herbicide methoxychlor is also produced from chloral.
Treating chloral with sodium hydroxide gives chloroform Cl3CH and sodium formate HCOONa.
- Cl3CCHO + NaOH → Cl3CH + HCOONa
Chloral is easily reduced to trichloroethanol, which is produced in the body from chloral.[3]
Safety
Chloral and chloral hydrate have the same properties biologically since the former hydrates rapidly. Chloral hydrate was routinely administered to patients on the gram scale with no lasting effects. Prolonged exposure to the vapors is unhealthy with a LC50 for 4-hour exposure of 440 mg/m3.[3]
See also
References
- See:
- Liebig, Justus (1832). "Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen" [On the compounds which arise by the reaction of chlorine with alcohol [ethanol], ether [diethyl ether], oil-forming gas [ethylene], and spirit of vinegar [acetone]]. Annalen der Pharmacie (in German). 1: 182–230. pp. 189–191 "Wirkung des Chlors auf Alkohol" [Reaction of Chlorine with alcohol [i.e., ethanol]] ; pp. 191–194 "Darstellung des Chloral[s]" [Preparation of chloral] ; pp. 195–198 "Eigenschaften des Chlorals" [Properties of chloral].
Liebig named chloral on p. 191. From p. 191: "Ich werde in dem Folgenden zeigen, dass bei einer vollkommnen Zersetzung des Alkohols das Chlor den Wasserstoff desselben abscheidet und diesen Wasserstoff ersetzt; es entsteht eine neue eigenthümliche Verbindung von Chlor, Kohlenstoff und Sauerstoff, welche ich, indem ich keinen zweckmässigeren Namen weiss, vorläufig Chloral nenne. Dieser Name ist dem Worte Aethal nachgebildet." (In the following, I will show that during a complete breakdown of ethanol, chlorine removes its [i.e., ethanol's] hydrogen and replaces this hydrogen; there arises a strange new compound of chlorine, carbon, and oxygen, which I — as I know no more appropriate name — provisionally name "chloral". This name is patterned after the word Aethal [i.e., ethyl].) - Reprinted in: Liebig, Justus (1832). ""Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen"" [On the compounds which arise by the reaction of chlorine with alcohol [ethanol], ether [diethyl ether], oil-forming gas [ethylene], and spirit of vinegar [acetone]]. Annalen der Physik und Chemie. 2nd series (in German). 24: 243–295. pp. 250-252 "Wirkung des Chlors auf Alkohol" [Reaction of Chlorine with alcohol [i.e., ethanol]] ; pp. 252–255 "Darstellung des Chloral[s]" [Preparation of chloral] ; pp. 255–259 "Eigenschaften des Chlorals" [Properties of chloral].
- Gmelin, Leopold, ed. (1848). Handbuch der Chemie (in German). vol. 4 (4th ed.). Heidelberg, [Germany]: Karl Winter. pp. 893–897.
- Liebig, Justus (1832). "Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen" [On the compounds which arise by the reaction of chlorine with alcohol [ethanol], ether [diethyl ether], oil-forming gas [ethylene], and spirit of vinegar [acetone]]. Annalen der Pharmacie (in German). 1: 182–230. pp. 189–191 "Wirkung des Chlors auf Alkohol" [Reaction of Chlorine with alcohol [i.e., ethanol]] ; pp. 191–194 "Darstellung des Chloral[s]" [Preparation of chloral] ; pp. 195–198 "Eigenschaften des Chlorals" [Properties of chloral].
- Liebig passed dry chlorine gas through anhydrous ethanol for 11–13 days, until hydrogen chloride ceased to form. The product was dried by shaking with concentrated sulfuric acid, decanted over chalk, and then distilled. (Liebig, 1832), pp. 191–194.
- Jira, Reinhard; Kopp, Erwin; McKusick, Blaine C.; Röderer, Gerhard; Bosch, Axel; Fleischmann, Gerald. "Chloroacetaldehydes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_527.pub2.
- Zeidler, Othmar (1874). "Verbindungen von Chloral mit Brom- und Chlorbenzol" [Compounds of chloral with bromo- and chlorobenzene]. Berichte der deutschen chemischen Gesellschaft. 7 (2): 1180–1181. doi:10.1002/cber.18740070278.