Remimazolam
Remimazolam, sold under the brand name Byfavo, is a medication for the induction and maintenance of procedural sedation in adults lasting 30 minutes or less.[2][3] is a benzodiazepine derivative drug, developed by PAION, in collaboration with Japanese licensee Ono Pharmaceutical as an alternative to the short-acting imidazobenzodiazepine midazolam, for use in induction of anaesthesia and conscious sedation for minor invasive procedures. Remimazolam was found to be both faster acting and shorter lasting than midazolam, and human clinical trials showed a faster recovery time and predictable, consistent pharmacokinetics, suggesting some advantages over existing drugs for these applications.[4][5]
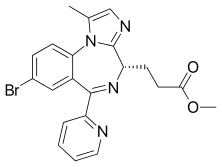 | |
| Clinical data | |
|---|---|
| Trade names | Byfavo |
| Other names | CNS-7056[1] |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H19BrN4O2 |
| Molar mass | 439.313 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The most common side effects for procedural sedation include hypotension (low blood pressure), hypertension (high blood pressure), diastolic hypertension, systolic hypertension, hypoxia (low blood oxygen level), and diastolic hypotension.[2][3]
Remimazolam was approved for medical use in the United States in July 2020.[2][3]
Medical uses
Remimazolam is indicated for the induction and maintenance of procedural sedation in adults lasting 30 minutes or less.[2][3][6]
History
Remimazolam was approved for medical use in the United States in July 2020.[2][3]
The U.S. Food and Drug Administration (FDA) approved remimazolam based on evidence from three clinical trials (Trial 1/NCT02290873, Trial 2/NCT02296892 and Trial 3/NCT02532647) in adults undergoing short procedures.[2] Trials were conducted at 32 sites in the United States.[2]
Trials 1 and 3 were conducted in participants undergoing colonoscopy and Trial 2 was conducted in participants undergoing bronchoscopy procedures.[2]
In the trials, participants were randomly divided in three groups: one group received remimazolam, one group received placebo and one group received midazolam (similar, but approved drug).[2] In the first two groups, neither participants nor investigators knew which medications were given and participants could also receive midazolam as a rescue drug when needed for sedation.[2] In the third group, all participants received midazolam only[2] Additionally, in all three trials participants received a medication for pain control[2]
Trials 1 and 2 compared participants who received remimazolam to participants in the other two groups, measuring the success of sedation with the set of pre-determined criteria.[2] Data from Trial 3 were used primarily to assess the side effects of remimazolam when multiple dosing is used.[2]
Research
Phase I[7] and Ib[8] dose-finding studies for procedural sedation with patients recovering faster from remimazolam than midazolam. Phase II trials comparing remimazolam to the standard anesthesia protocols for cardiac surgery and colonoscopy were presented at major conferences in October 2014.[9]
A Phase IIa trial comparing remimazolam to midazolam for upper endoscopy was published in December 2014, finding a similar safety profile.[10] Remimazolam was originally discovered in the late 1990s at Glaxo Wellcome in their labs in Research Triangle Park, North Carolina.
References
- Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. (July 2007). "CNS 7056: a novel ultra-short-acting Benzodiazepine". Anesthesiology. 107 (1): 60–6. doi:10.1097/01.anes.0000267503.85085.c0. ISSN 0003-3022. PMID 17585216. S2CID 19504961.
- "Drug Trials Snapshots: Byfavo". U.S. Food and Drug Administration (FDA). 2 July 2020. Retrieved 21 July 2020.

- "Cosmo Pharmaceuticals Announces US FDA Approval of Byfavo (remimazolam injection) for the Induction and Maintenance of Procedural Sedation". Cosmo Pharmaceuticals NV (Press release). 2 July 2020. Retrieved 2 July 2020.
- Rogers WK, McDowell TS (December 2010). "Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures". IDrugs : The Investigational Drugs Journal. 13 (12): 929–37. PMID 21154153.
- Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT (March 2011). "Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology". Pharmacological Reviews. 63 (1): 243–67. doi:10.1124/pr.110.002717. PMID 21245208. S2CID 930379.
- "Byfavo: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 2 July 2020.
- Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM (August 2012). "A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics". Anesthesia and Analgesia. 115 (2): 274–83. doi:10.1213/ANE.0b013e31823f0c28. PMID 22190555. S2CID 21457057.
- Worthington, Mark T.; Antonik, Laurie J.; Goldwater, D. Ronald; Lees, James P.; Wilhelm-Ogunbiyi, Karin; Borkett, Keith M.; Mitchell, Mack C. (Nov 2013). "A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy". Anesthesia & Analgesia. 117 (5): 1093–100. doi:10.1213/ANE.0b013e3182a705ae. PMID 24108261. S2CID 24581168.
- "Two Scientific Remimazolam Presentations Are Accepted for ASA and ACG Meeting in October 2014". MarketWired. Oct 1, 2014. Retrieved 2014-10-24.
- Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, et al. (April 2015). "A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy". Anesthesia & Analgesia. 120 (4): 771–80. doi:10.1213/ANE.0000000000000548. PMID 25502841. S2CID 20941788.
External links
- "Remimazolam". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02290873 for "A Phase III Study of the Efficacy and Safety of Remimazolam Compared to Placebo and Midazolam in Colonoscopy Patients" at ClinicalTrials.gov
- Clinical trial number NCT02296892 for "A Phase III Study of Remimazolam in Patients Undergoing Bronchoscopy" at ClinicalTrials.gov
- Clinical trial number NCT02532647 for "Safety and Efficacy of Remimazolam in ASA III and IV Patients Undergoing Colonoscopy" at ClinicalTrials.gov