Spirobarbital
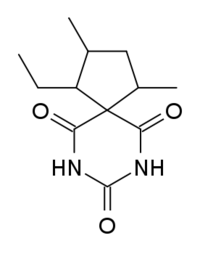
Spirobarbital is a barbiturate derivative developed by Eli Lilly in the 1940s.[1] It has hypnotic and sedative effects, and has a moderate potential for abuse.[2]
 | |
| Clinical data | |
|---|---|
| Other names | 5-spiro-(2'-ethyl-3'-5'-dimethyl-cyclopentyl)barbituric acid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H18N2O3 |
| Molar mass | 238.287 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
References
- US Patent 2561688
- Isbell H, Chrusciel TS. Dependence Liability of Non-Narcotic Drugs. Bulletin of the World Health Organization 1970; 43: Supplement.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.