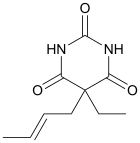
Crotylbarbital
Crotylbarbital (Mepertan, Kalipnon, Barotal), also known as crotarbital, is a barbiturate derivative developed by Eli Lilly in the 1930s[1]m It has sedative and hypnotic effects,[2] and was used for the treatment of insomnia until it was replaced by newer alternative drugs with less side effects and lower risk of overdose.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.162 |
| Chemical and physical data | |
| Formula | C10H14N2O3 |
| Molar mass | 210.233 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
See also
References
- US Patent 2250422
- Walther T, Meyer FP, Puchta K, Walther H. Effect of an acute dose of crotylbarbital on reaction time and attention testing in healthy human subjects. International Journal of Clinical Pharmacology, Therapy and Toxicology. 1983 Jun;21(6):306-10.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.