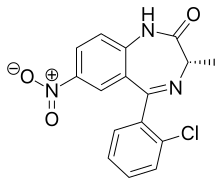
Meclonazepam
Meclonazepam[1] ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam.[2] It has sedative and anxiolytic actions like those of other benzodiazepines,[3] and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.[4]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H12ClN3O3 |
| Molar mass | 329.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Meclonazepam was never used as medicine and instead appeared online as a designer drug.[5][6][7]
Legal Issues
United Kingdom
In the UK, meclonazepam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[8]
gollark: People will probably complain if their package delivery gets electrolasered and electroned.
gollark: Don't electrons repel each other?
gollark: But I *need* atmosphere!
gollark: How well do said electron beams work at a really large distance?
gollark: So either launch it from just a railgun or something, and have some way to decelerate it enough that it doesn't wreck the parcel on landing, or have it land sensibly and either fly back or get mailed back.
See also
References
- U.S. Patent 4,031,078
- The Lundbeck Institute. "Meclonazepam". Psychotropics. Lundbeck.
- Ansseau, M.; Doumont, A.; Thiry, D.; Von Frenckell, R.; Collard, J. (1985). "Initial study of methylclonazepam in generalized anxiety disorder. Evidence for greater power in the cross-over design". Psychopharmacology. 87 (2): 130–135. doi:10.1007/bf00431795. PMID 3931136.
- O'Boyle, C.; Lambe, R.; Darragh, A. (1985). "Central Effects in Man of the Novel Schistosomicidal Benzodiazepine Meclonazepam". European Journal of Clinical Pharmacology. 29 (1): 105–108. doi:10.1007/bf00547377. PMID 4054198.
- Markus R. Meyer; Madeleine Pettersson Bergstrand; Anders Helander; Olof Beck (May 2016). "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry. 408 (13): 3571–3591. doi:10.1007/s00216-016-9439-6. PMID 27071765.
- Pettersson Bergstrand M, Helander A, Hansson T, Beck O (2016). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Test Anal. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- Manchester, Kieran R.; Maskell, Peter D.; Waters, Laura (2018). "Experimental versus theoretical log D7.4, pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances" (PDF). Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. ISSN 1942-7611. PMID 29582576.
- "The Misuse of Drugs Act 1971 (Amendment) Order 2017".
Further reading
- Abdul-Ghani, R. A.; Loutfy, N.; Hassan, A. (2009). "Experimentally promising antischistosomal drugs: A review of some drug candidates not reaching the clinical use". Parasitology Research. 105 (4): 899–906. doi:10.1007/s00436-009-1546-2. PMID 19588166.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.