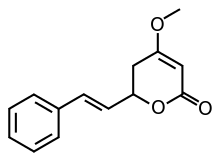
Kavain
Kavain is the main kavalactone found mostly in the roots of the kava plant.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methoxy-6-[(E)-2-phenylethenyl]-5,6-dihydro-2H-pyran-2-one | |
| Other names
(E)-4-Methoxy-6-styryl-5,6-dihydro-2H-pyran-2-one Kawain | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.189 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H14O3 | |
| Molar mass | 230.263 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pharmacology
Kavain has anticonvulsive properties, attenuating vascular smooth muscle contraction through interactions with voltage-dependent Na+ and Ca2+ channels.[1] How this effect is mediated and to what extent this mechanism is involved in the anxiolytic and analgesic effects of kavalactones on the central nervous system is unknown. Kavain's pharmacological activities have not been sufficiently investigated, and neither its effect as a serotonin reuptake inhibitor nor its monoamine (norepinephrine) uptake inhibitions and activation of NMDA receptors have been confirmed.
The mechanism behind the psychotropic, sedative, and anxiolytic actions of kavain and related kavalactones is still debated. Direct binding to the benzodiazepine/flumazenil binding site of the GABA-A receptor does not occur with kavain enantiomers.[2] Many studies involved kava extracts from different plant parts and are, therefore, not applicable to kavain itself. In 2016, kavain was shown to bind at the α4β2δ GABAA receptor and potentiate GABA efficacy.[3]
A comparative review of in-vivo studies with kavain (and related kavapyrones) to commonly used antiepileptic drugs and mood stabilizers affecting ion fluxes indicates that the kavapyrones are weakly Na+ antagonistic and therefore antiepileptic. They also have pronounced L- type Ca2+ channel antagonistic properties and act as a positive modulator of the early K+ outward current, which contribute to mood stabilizing properties similar to lamotrigine.[4]
Kavain and analogs remain interesting for drug discovery against a variety of cellular targets, including P-glycoprotein (Pgp), cytochrome P450, and cyclo-oxygenase (COX) enzymes among others.[5]
See also
References
- Bradić, I; Pasini, M (1975). "Hirschsprung's disease -- therapy and results". Acta Chirurgica Iugoslavica. 22 (2): 183–95. PMID 1235738.
- Boonen, Georg; Häberlein, Hans (2007). "Influence of Genuine Kavapyrone Enantiomers on the GABAABinding Site". Planta Medica. 64 (6): 504–6. doi:10.1055/s-2006-957502. PMID 9776662.
- Chua HC, Christensen ET, Hoestgaard-Jensen K, Hartiadi LY, Ramzan I, Jensen AA, Absalom NL, Chebib M (2016). "Kavain, the Major Constituent of the Anxiolytic Kava Extract, Potentiates GABAA Receptors: Functional Characteristics and Molecular Mechanism". PLoS ONE. 11 (6): e0157700. Bibcode:2016PLoSO..1157700C. doi:10.1371/journal.pone.0157700. PMC 4917254. PMID 27332705.
- Grunze, Heinz; Langosch, Jens; Schirrmacher, Karin; Bingmann, Dieter; Von Wegerer, Jörg; Walden, Jörg (2001). "Kava pyrones exert effects on neuronal transmission and transmembraneous cation currents similar to established mood stabilizers - a review". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 25 (8): 1555–70. doi:10.1016/S0278-5846(01)00208-1. PMID 11642654.
- Rowe, A.; Narlawar, R.; w. Groundwater, P.; Ramzan, I. (2011). "Kavalactone Pharmacophores for Major Cellular Drug Targets". Mini Reviews in Medicinal Chemistry. 11 (1): 79–83. doi:10.2174/138955711793564088. PMID 21034404.