Ocinaplon
Ocinaplon is an anxiolytic drug in the pyrazolopyrimidine family of drugs. Other pyrazolopyrimidine drugs include zaleplon and indiplon.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H11N5O |
| Molar mass | 301.309 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ocinaplon has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and relatively little sedative or amnestic effect.[1]
Medical uses
A 2019 review found tentative evidence of benefit in anxiety.[2]
Mechanism of action
The mechanism of action by which ocinaplon produces its anxiolytic effects is by modulating GABAA receptors,[3] although ocinaplon is more subtype-selective than most benzodiazepines.[4]
Availability
Development of ocinaplon is discontinued due to liver complications that occurred in one of the Phase III subjects.[5]
Synthesis
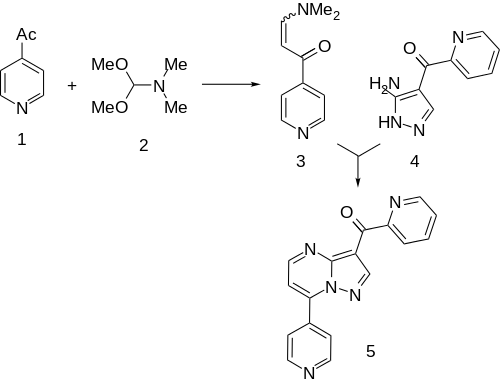
Condensation of 4-Acetylpyridine[8] with N,N-Dimethylformamide dimethyl acetal (DMFDMA) gives the "enamide" (3). This is then condensed with (3-Amino-1H-pyrazol-4-yl)(2-pyridinyl)methanone (4) (96219-90-8).[9][10] This is the same intermediate as was used in the synthesis of zaleplon in which the nitrile is replaced by a 2-acetylpyridil moiety. This affords the anxiolytic agent ocinaplon (5).
References
- Lippa A, Czobor P, Stark J, Beer B, Kostakis E, Gravielle M, et al. (May 2005). "Selective anxiolysis produced by ocinaplon, a GABA(A) receptor modulator". Proceedings of the National Academy of Sciences of the United States of America. 102 (20): 7380–5. Bibcode:2005PNAS..102.7380L. doi:10.1073/pnas.0502579102. PMC 1129138. PMID 15870187.
- Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N (February 2019). "Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis". Lancet. 393 (10173): 768–777. doi:10.1016/S0140-6736(18)31793-8. PMID 30712879.
- Mirza NR, Rodgers RJ, Mathiasen LS (March 2006). "Comparative cue generalization profiles of L-838, 417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination". The Journal of Pharmacology and Experimental Therapeutics. 316 (3): 1291–9. doi:10.1124/jpet.105.094003. PMID 16339395.
- Atack JR (May 2005). "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opinion on Investigational Drugs. 14 (5): 601–18. doi:10.1517/13543784.14.5.601. PMID 15926867.
- "DOV Pharmaceutical, Inc. Places Ocinaplon Phase III Clinical Trial On Hold". PR NewsWire. Archived from the original on 4 March 2016.
- Baumann M, Baxendale IR (October 2013). "An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles". Beilstein Journal of Organic Chemistry. 9: 2265–319. doi:10.3762/bjoc.9.265. PMC 3817479. PMID 24204439.
- ARKIVOC 2010 (ii) 267-282
- LaMattina JL, Sulesk RT (1986). "A-Amino Acetals: 2,2-Diethoxy-2-(4-Pyridyl)Ethylamine". Organic Syntheses. 64: 19. doi:10.15227/orgsyn.064.0019.
- U.S. Patent 4,900,836
- CA 1243029