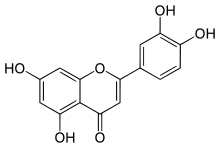
Luteolin
Luteolin is a flavone, a type of flavonoid, with a yellow crystalline appearance.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4-Dihydroxyphenyl)- 5,7-dihydroxy-4-chromenone | |
| Other names
Luteolol Digitoflavone Flacitran Luteoline 3′,4′,5,7-Tetrahydroxyflavone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.038 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O6 | |
| Molar mass | 286.239 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Luteolin is the principal yellow dye compound that is obtained from the plant Reseda luteola, which has been used as a source of the dye since at least the first millennium B.C. Luteolin was first isolated in pure form, and named, in 1829 by the French chemist Michel Eugène Chevreul.[2][3][4] Luteolin's empirical formula was determined by the Austrian chemists Heinrich Hlasiwetz and Leopold Pfaundler in 1864.[5][6] In 1896, the English chemist Arthur George Perkin proposed the correct structure for luteolin.[7] Perkin's proposed structure for luteolin was confirmed in 1900 when the Polish-Swiss chemist Stanislaw Kostanecki (1860–1910) and his students A. Różycki and J. Tambor synthesized luteolin.[8][9]
Natural occurrences
Luteolin is most often found in leaves, but it is also seen in rinds, barks, clover blossom, and ragweed pollen.[1] It has also been isolated from the aromatic flowering plant, Salvia tomentosa in the mint family, Lamiaceae.[10]
Dietary sources include celery, broccoli, green pepper, parsley, thyme, dandelion, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano.[11][12] It can also be found in the seeds of the palm Aiphanes aculeata.[13]
References
- Mann, John (1992). Secondary Metabolism (2nd ed.). Oxford, UK: Oxford University Press. pp. 279–280. ISBN 978-0-19-855529-2.
- Chevreul, M.E. (1829). "30e Leçon, Chapitre XI. De la Gaude. [30th lesson. Chapter 11. On Weld (i.e., the plant Reseda luteola, which provides a yellow dye)]". Leçons de Chimie Appliquée à la Teinture [Lessons on Chemistry Applied to Dyeing] (in French). Paris, France: Pichon et Didier. pp. 143–148. Chevreul named luteolin on p. 144: "J'ai fait des recherches sur la composition de la gaude, j'ai obtenu le principe colorant critalisé par sublimation; je l'ai nommé lutéolin." (I have done some research on the composition of weld; I obtained the principal colorant [which I] crystallized via sublimation; I have called it "luteolin".)
- Thomson, Thomas (1838). Chemistry of Organic Bodies. Vegetables. London, England: J.B. Baillière. pp. 415–416.
- However, Perkin claimed (without citing a source) that Chevreul had isolated luteolin as early as 1814–1815. See: Perkin, Arthur George; Everest, Arthur Ernest (1918). The Natural Organic Colouring Matters. London, England: Longmans, Green and Co. p. 4.
- Hlasiwetz, H.; Pfaundler, L. (1864). "Über das Morin, Maclurin und Quercitrin". Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe. (Part 2) (in German). 50: 6–59. ; see pp. 44–45.
- Hlasiwetz, H.; Pfaundler, L. (1865). "Ueber das Morin, Maclurin und Quercitrin". Journal für praktische Chemie (in German). 94: 65–106. Hlasiwetz and Pfaundler melted quercitrin with potassium carbonate. Among the reaction's products, they found paradatiscetin, whose empirical formula they determined to be C15H10O6 (p. 94). They concluded that although luteolin and paradatiscetin were isomeric (i.e., had the same empirical formula), they were distinct compounds. From p. 94: "Das Luteolin scheint demnach wohl als isomer oder metamer mit unserer Substanz betrachtet werden zu können. Eine Identität beider liegt jedoch nicht vor, denn an einer Probe Luteolin fanden wir die charakteristischen Farben-reactionen nicht, welche das Paradatiscetin kaum verwechseln lassen." (Luteolin thus seems to be able to be regarded perhaps as an isomer or metamer of our substance [viz, paradatiscetin]. However, the two are evidently not identical, for upon a test of luteolin, we did not find the characteristic color reactions, which hardly allows paradatiscetin to be confused [with it].)
- Perkin, A. G. (1896). "Luteolin. Part II". Journal of the Chemical Society. 69: 799–803. See p. 803.
- Kostanecki, St. v.; Róžycki, A.; Tambor, J. (1900). "Synthese des Luteolins". Berichte der Deutschen Chemischen Gesellschaft (in German). 33: 3410–3417.
- Thorpe, Edward, ed. (1913). A Dictionary of Applied Chemistry. vol. 5. London, England: Longmans, Green, and Co. pp. 747–748.
- A. Ulubelen; M. Miski; P. Neuman; T. J. Mabry (1979). "Flavonoids of Salvia tomentosa (Labiatae)". Journal of Natural Products. 42 (4): 261–3. doi:10.1021/np50003a002.
- Kayoko Shimoi; Hisae Okada; Michiyo Furugori; Toshinao Goda; Sachiko Takase; Masayuki Suzuki; Yukihiko Hara; Hiroyo Yamamoto; Naohide Kinae (1998). "Intestinal absorption of luteolin and luteolin 7-O-[beta]-glucoside in rats and humans". FEBS Letters. 438 (3): 220–4. doi:10.1016/S0014-5793(98)01304-0. PMID 9827549.
- López-Lázaro M. (2009). "Distribution and biological activities of the flavonoid luteolin". Mini Rev Med Chem. 9 (1): 31–59. doi:10.2174/138955709787001712. PMID 19149659.
- Lee, D; Cuendet, M; Vigo, JS; Graham, JG; Cabieses, F; Fong, HH; Pezzuto, JM; Kinghorn, AD (2001). "A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata". Organic Letters. 3 (14): 2169–71. doi:10.1021/ol015985j. PMID 11440571.