TBX3
T-box transcription factor TBX3 is a protein that in humans is encoded by the TBX3 gene.[1][2]
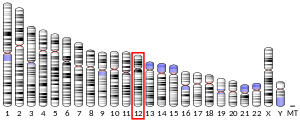
T-box 3 (TBX3) is a member of the T-box gene family of transcription factors which all share a highly conserved DNA binding domain known as the T-box. The T-box gene family consists of 17 members in mouse and humans that are grouped into five subfamilies, namely Brachyury (T), T-brain (Tbr1), TBX1, TBX2, and TBX6. Tbx3 is a member of the Tbx2 subfamily which includes Tbx2, Tbx4 and Tbx5.[3] The human TBX3 gene maps to chromosome 12 at position 12q23-24.1 and consists of 7 exons which encodes a 723 amino acid protein (ENSEMBL assembly release GRCh38.p12).
Transcript splicing
Alternative processing and splicing results in at least 4 distinct TBX3 isoforms with TBX3 and TBX3+2a being the predominant isoforms. TBX3+2a results from alternative splicing of the second intron which leads to the addition of the +2a exon and consequently this isoform has an additional 20 amino acids within the T-box DNA binding domain.[8][9] The functions of TBX3 and TBX3+2a may vary slightly across different cell types.[9][10][11][12][13][14]
Structure and function
TBX3 has domains which are important for its transcription factor function which include a DNA-binding domain (DBD) also called the T-box, a nuclear localization signal, two repression domains (R2 and R1) and an activation domain (A).[15] The T-box recognizes a palindromic DNA sequence (T(G/C)ACACCT AGGTGTGAAATT) known as the T-element, or half sites within this sequence called half T-elements, although it can also recognize variations within the consensus T-element sequences. While there are 29 predicted phosphorylation sites in the TBX3 protein only the SP190, SP692 and S720 have been fully characterized. The kinases involved are cyclin A-CDK2 at either SP190 or SP354, p38 mitogen-activated protein (MAP) kinase at SP692 in embryonic kidney cells and AKT3 at S720 in melanoma. These modifications act in a context dependent manner to promote TBX3 protein stability, nuclear localization and transcriptional activity.[16][17][18]
TBX3 can activate and/or repress its target genes by binding a T-element, or half T-element sites.[19] Indeed, Tbx3 binds highly conserved T-elements to activate the promoters of Eomes, T, Sox17 and Gata6, which are factors essential for mesoderm differentiation and extra embryonic endodermal.[20][21] Furthermore, in the cancer context, TBX3 directly represses the cell cycle regulators p19ARF/p14ARF [22] , p21WAF1 [23] and TBX2 [24] as well as E-cadherin [11] which encodes a cell adhesion molecule, to promote proliferation and migration. TBX3 directly represses a region of the PTEN promoter which lacks putative T-elements, but which forms an important regulatory unit for PTEN transcriptional activators, thus raising the possibility that TBX3 may also repress some of its target genes through interfering with transcriptional activators.[25]
The function of TBX3 as either a transcriptional repressor or transcriptional activator is, in part, modulated by protein co-factors. For example, it can interact with other transcription factors such as Nkx2-5, Msx 1/2 [26] and Sox4 [27] to assist it binding to its target genes to regulate heart development [10][28][29][30][31] and it can interact with histone deacetylases (HDACs) 1, 2, 3 and 5 to repress p14ARF in breast cancer and with HDAC5 to repress E-cadherin to promote metastasis in hepatocellular carcinoma.[32][33] Lastly, TBX3 can also co-operate with other factors to inhibit the process of mRNA splicing by directly binding RNAs containing the core motif of a T-element.[10][11][12][13][14] Indeed, TBX3 interacts with Coactivator of AP1 and Estrogen Receptor (CAPERα) to repress the long non-coding RNA, Urothelial Cancer Associated 1 (UCA1), which leads to the bypass of senescence through the stabilization of p16INK4a mRNA.[34]
Role in development
During mouse embryonic development, Tbx3 is expressed in the inner cell mass of the blastocyst, in the extraembryonic mesoderm during gastrulation, and in the developing heart, limbs,[35] musculoskeletal structures,[36] mammary glands,[37] nervous system,[38] skin,[39] eye,[40] liver,[41] pancreas,[42] lungs [43] and genitalia.[8] Tbx3 null embryos show defects in, among other structures, the heart, mammary glands and limbs and they die in utero by embryonic day E16.5, most likely due to yolk sac and heart defects. These observations together with numerous other studies have illustrated that Tbx3 plays crucial roles in the development of the heart,[44] mammary glands,[45] limbs [46] and lungs.[47]
Role in stem cells
Embryonic stem cells (ESCs) and adult stem cells, are undifferentiated cells which when they divide have the potential to either remain a stem cell or to differentiate into other specialized cells. Adult stem cells are multipotent progenitor cells found in numerous adult tissues and, as part of the body repair system, they can develop into more than one cell type but they are more limited than ESCs.[48] TBX3 is highly expressed in mouse ESCs (mESCs) and appears to have a dual role in these cells. Firstly it can enhance and maintain stem cell pluripotency by preventing differentiation and enhancing self-renewal and secondly it can maintain the pluripotency and differentiation potential of mESCS.[49][50] Induced pluripotent stem cells (iPSCs) are ESC-like cells that can generate scalable quantities of relevant tissue and are of major interest for their application in personalized regenerative medicine, drug screening, and for our understanding of the cell signaling networks that regulate embryonic development and disease. In vitro studies have shown that Tbx3 is an important factor that, together with KLF4, SOX2, OCT4, Nanog, LIN-28A and C-MYC, can reprogram somatic cells to form iPS cells.[51]
Clinical significance
TBX3 has been implicated in human diseases including the ulnar mammary syndrome,[52] obesity,[38] rheumatoid arthritis[53] and cancer.[54]
In humans, heterozygous mutations of TBX3 lead to the autosomal dominant developmental disorder, ulnar mammary syndrome (UMS), which is characterized by a number of clinical features including mammary and apocrine gland hypoplasia, upper limb defects, malformations of areola, dental structures, heart and genitalia.[8][55] Several UMS causing mutations in the TBX3 gene have been reported which include 5 nonsense, 8 frameshift (due to deletion, duplication and insertion), 3 missense and 2 splice site mutations. Missense mutations within the T-domain, or the loss of RD1 result in aberrant transcripts and truncated proteins of TBX3. These mutations lead to reduced DNA binding, transcriptional control and splicing regulation of TBX3 and the loss of function and are associated with the most severe phenotype of UMS.[22][56][57][58]
Tbx3 is expressed in heterogenous populations of hypothalamic arcuate nucleus neurons which control energy homeostasis by regulating appetite and energy expenditure and the ablation of TBX3 function in these neurons was shown to cause obesity in mouse models. Importantly, Tbx3 was shown to be a key player in driving the functional heterogeneity of hypothalamic neurons and this function was conserved in mice, drosophila and humans.[38] Genome wide association studies also causally linked TBX3 to rheumatoid arthritis (RA) susceptibility and a recent study identified Tbx3 as a candidate gene for RA in collagen-induced arthritis (CIA) mouse models.[53][59] The severity of RA directly correlated with TBX3 serum levels in the CIA mouse models. Furthermore, Tbx3 was shown to repress B lymphocyte proliferation and to activate the humoral immune response which is associated with chronic inflammation of the synovium leading to RA. Tbx3 may thus be an important player in regulating the immune system and could be used as a biomarker for the diagnosis of RA severity.[53]
TBX3 is overexpressed in a wide range of carcinomas (breast, pancreatic, melanoma, liver, lung, gastric, ovarian, bladder and head and neck cancers) and sarcomas (chondrosarcoma, fibrosarcoma, liposarcoma, rhabdomyosarcoma and synovial sarcoma) and there is compelling evidence that it contributes to several hallmarks of cancer. Indeed, TBX3 can bypass cellular senescence, apoptosis and anoikis as well as promote uncontrolled cell proliferation, tumor formation, angiogenesis and metastasis.[14][33][54][60][61][62] Furthermore, TBX3 contributes to the expansion of cancer stem cells (CSCs) and is a key player in regulating pluripotency-related genes in these cells. CSCs contribute to tumor relapse and drug resistance and thus this may be another mechanism by which TBX3 contributes to cancer formation and tumor aggressiveness.[63] The mechanisms by which TBX3 contributes to oncogenic processes involve, in part, its ability to inhibit the tumor suppressor pathways p14ARF/p53/p21WAF1/CIP1,[15][32][64] p16INK4a/pRb, p57KIP2,[65] PTEN,[25] E-cadherin[60][61] and activating the angiogenesis-associated genes FGF2 and VEGF-A[66] and the EMT gene SNAI.[14] Some of the oncogenic signaling molecules identified that upregulate TBX3 include TGF-β,[24][67] BRAF-MAPK,[68] c-Myc,[16] AKT,[18] and PLCᗴ/PKC.[69] The function of TBX3 is also regulated by phosphorylation by the p38-MAPK, AKT3 and cyclin A/CDK2[16] and by protein co-factors, which include PRC2,[65] Histone Deacetylases 1, 2, 3 and 5[32] and CAPERα.[34]
There is also evidence that TBX3 may function as a tumour suppressor. During oncogenesis, TBX3 is silenced by methylation in some cancers and this was associated with a poor overall survival, resistance to cancer therapy and a more invasive phenotype.[70][71][72][73] In addition, TBX3 is overexpressed in fibrosarcoma cells and removing TBX3 from these cells led to a more aggressive phenotype.[74]
References
- Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD (Jan 1997). "Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family". Nature Genetics. 15 (1): 21–9. doi:10.1038/ng0197-21. PMID 8988164.
- "Entrez Gene: TBX3 T-box 3 (ulnar mammary syndrome)".
- Papaioannou VE (October 2014). "The T-box gene family: emerging roles in development, stem cells and cancer". Development. 141 (20): 3819–33. doi:10.1242/dev.104471. PMC 4197708. PMID 25294936.
- GRCh38: Ensembl release 89: ENSG00000135111 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000018604 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, et al. (July 1997). "Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome". Nature Genetics. 16 (3): 311–5. doi:10.1038/ng0797-311. PMID 9207801.
- Fan W, Huang X, Chen C, Gray J, Huang T (August 2004). "TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines". Cancer Research. 64 (15): 5132–9. doi:10.1158/0008-5472.CAN-04-0615. PMID 15289316.
- Hoogaars WM, Barnett P, Rodriguez M, Clout DE, Moorman AF, Goding CR, Christoffels VM (June 2008). "TBX3 and its splice variant TBX3 + exon 2a are functionally similar". Pigment Cell & Melanoma Research. 21 (3): 379–87. doi:10.1111/j.1755-148X.2008.00461.x. PMID 18444963.
- Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR (October 2008). "Tbx3 represses E-cadherin expression and enhances melanoma invasiveness". Cancer Research. 68 (19): 7872–81. doi:10.1158/0008-5472.CAN-08-0301. PMID 18829543.
- Zhao D, Wu Y, Chen K (February 2014). "Tbx3 isoforms are involved in pluripotency maintaining through distinct regulation of Nanog transcriptional activity". Biochemical and Biophysical Research Communications. 444 (3): 411–4. doi:10.1016/j.bbrc.2014.01.093. PMID 24472544.
- Krstic M, Macmillan CD, Leong HS, Clifford AG, Souter LH, Dales DW, et al. (August 2016). "The transcriptional regulator TBX3 promotes progression from non-invasive to invasive breast cancer". BMC Cancer. 16 (1): 671. doi:10.1186/s12885-016-2697-z. PMC 4994202. PMID 27553211.
- Krstic M, Kolendowski B, Cecchini MJ, Postenka CO, Hassan HM, Andrews J, et al. (June 2019). "TBX3 promotes progression of pre-invasive breast cancer cells by inducing EMT and directly up-regulating SLUG". The Journal of Pathology. 248 (2): 191–203. doi:10.1002/path.5245. PMC 6593675. PMID 30697731.
- Carlson H, Ota S, Song Y, Chen Y, Hurlin PJ (May 2002). "Tbx3 impinges on the p53 pathway to suppress apoptosis, facilitate cell transformation and block myogenic differentiation". Oncogene. 21 (24): 3827–35. doi:10.1038/sj.onc.1205476. PMID 12032820.
- Willmer T, Peres J, Mowla S, Abrahams A, Prince S (2015-10-02). "The T-Box factor TBX3 is important in S-phase and is regulated by c-Myc and cyclin A-CDK2". Cell Cycle. 14 (19): 3173–83. doi:10.1080/15384101.2015.1080398. PMC 4825571. PMID 26266831.
- Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, et al. (April 2011). "Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling". The Journal of Cell Biology. 193 (2): 319–32. doi:10.1083/jcb.201009100. PMC 3080255. PMID 21482718.
- Peres J, Mowla S, Prince S (January 2015). "The T-box transcription factor, TBX3, is a key substrate of AKT3 in melanomagenesis". Oncotarget. 6 (3): 1821–33. doi:10.18632/oncotarget.2782. PMC 4359334. PMID 25595898.
- Wilson V, Conlon FL (2002). "The T-box family". Genome Biology. 3 (6): REVIEWS3008. doi:10.1186/gb-2002-3-6-reviews3008. PMC 139375. PMID 12093383.
- Weidgang CE, Russell R, Tata PR, Kühl SJ, Illing A, Müller M, et al. (September 2013). "TBX3 Directs Cell-Fate Decision toward Mesendoderm". Stem Cell Reports. 1 (3): 248–65. doi:10.1016/j.stemcr.2013.08.002. PMC 3849240. PMID 24319661.
- Lu R, Yang A, Jin Y (March 2011). "Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells". The Journal of Biological Chemistry. 286 (10): 8425–36. doi:10.1074/jbc.M110.202150. PMC 3048727. PMID 21189255.
- Lingbeek ME, Jacobs JJ, van Lohuizen M (July 2002). "The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site in the initiator". The Journal of Biological Chemistry. 277 (29): 26120–7. doi:10.1074/jbc.M200403200. PMID 12000749.
- Willmer T, Hare S, Peres J, Prince S (March 2016). "The T-box transcription factor TBX3 drives proliferation by direct repression of the p21(WAF1) cyclin-dependent kinase inhibitor". Cell Division. 11 (1): 6. doi:10.1186/s13008-016-0019-0. PMC 4840944. PMID 27110270.
- Li J, Ballim D, Rodriguez M, Cui R, Goding CR, Teng H, Prince S (December 2014). "The anti-proliferative function of the TGF-β1 signaling pathway involves the repression of the oncogenic TBX2 by its homologue TBX3". The Journal of Biological Chemistry. 289 (51): 35633–43. doi:10.1074/jbc.M114.596411. PMC 4271245. PMID 25371204.
- Burgucu D, Guney K, Sahinturk D, Ozbudak IH, Ozel D, Ozbilim G, Yavuzer U (October 2012). "Tbx3 represses PTEN and is over-expressed in head and neck squamous cell carcinoma". BMC Cancer. 12 (1): 481. doi:10.1186/1471-2407-12-481. PMC 3517435. PMID 23082988.
- Boogerd KJ, Wong LY, Christoffels VM, Klarenbeek M, Ruijter JM, Moorman AF, Barnett P (June 2008). "Msx1 and Msx2 are functional interacting partners of T-box factors in the regulation of Connexin43". Cardiovascular Research. 78 (3): 485–93. doi:10.1093/cvr/cvn049. PMID 18285513.
- Boogerd CJ, Wong LY, van den Boogaard M, Bakker ML, Tessadori F, Bakkers J, et al. (December 2011). "Sox4 mediates Tbx3 transcriptional regulation of the gap junction protein Cx43". Cellular and Molecular Life Sciences. 68 (23): 3949–61. doi:10.1007/s00018-011-0693-7. PMC 3214269. PMID 21538160.
- Bakker ML, Boukens BJ, Mommersteeg MT, Brons JF, Wakker V, Moorman AF, Christoffels VM (June 2008). "Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system". Circulation Research. 102 (11): 1340–9. doi:10.1161/circresaha.107.169565. PMID 18467625.
- Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, et al. (July 2000). "Chamber formation and morphogenesis in the developing mammalian heart". Developmental Biology. 223 (2): 266–78. doi:10.1006/dbio.2000.9859. PMID 10882515.
- Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M (April 2004). "T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers". Developmental Dynamics. 229 (4): 763–70. doi:10.1002/dvdy.10487. PMID 15042700.
- Stennard FA, Harvey RP (November 2005). "T-box transcription factors and their roles in regulatory hierarchies in the developing heart". Development. 132 (22): 4897–910. doi:10.1242/dev.02099. PMID 16258075.
- Yarosh W, Barrientos T, Esmailpour T, Lin L, Carpenter PM, Osann K, et al. (February 2008). "TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases". Cancer Research. 68 (3): 693–9. doi:10.1158/0008-5472.can-07-5012. PMID 18245468.
- Dong L, Lyu X, Faleti OD, He ML (September 2018). "The special stemness functions of Tbx3 in stem cells and cancer development". Seminars in Cancer Biology. 57: 105–110. doi:10.1016/j.semcancer.2018.09.010. PMID 30268432.
- Kumar PP, Emechebe U, Smith R, Franklin S, Moore B, Yandell M, et al. (May 2014). "Coordinated control of senescence by lncRNA and a novel T-box3 co-repressor complex". eLife. 3. doi:10.7554/elife.02805. PMC 4071561. PMID 24876127.
- Tümpel, S (2002-10-15). "Regulation of Tbx3 Expression by Anteroposterior Signalling in Vertebrate Limb Development". Developmental Biology. 250 (2): 251–262. doi:10.1016/s0012-1606(02)90762-1. ISSN 0012-1606.
- Rao SB, Dinakar I, Rao KS (December 1971). "Giant intracranial epidural meningioma". Journal of Neurosurgery. 35 (6): 748–50. doi:10.3171/jns.1971.35.6.0748. PMID 5117227.
- Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, Kim HJ, et al. (November 2006). "Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development". Proceedings of the National Academy of Sciences of the United States of America. 103 (45): 16788–93. Bibcode:2006PNAS..10316788C. doi:10.1073/pnas.0604645103. PMC 1636533. PMID 17071745.
- Quarta C, Fisette A, Xu Y, Colldén G, Legutko B, Tseng YT, Reim A, Wierer M, De Rosa MC, Klaus V, Rausch R (2019-01-28). "Functional identity of hypothalamic melanocortin neurons depends on Tbx3". Nature Metabolism. 1 (2): 222–235. doi:10.1038/s42255-018-0028-1.
- Ichijo R, Kobayashi H, Yoneda S, Iizuka Y, Kubo H, Matsumura S, et al. (September 2017). "Tbx3-dependent amplifying stem cell progeny drives interfollicular epidermal expansion during pregnancy and regeneration". Nature Communications. 8 (1): 508. Bibcode:2017NatCo...8..508I. doi:10.1038/s41467-017-00433-7. PMC 5593911. PMID 28894084.
- Motahari Z, Martinez-De Luna RI, Viczian AS, Zuber ME (October 2016). "Tbx3 represses bmp4 expression and, with Pax6, is required and sufficient for retina formation". Development. 143 (19): 3560–3572. doi:10.1242/dev.130955. PMC 5087613. PMID 27578778.
- Suzuki A, Sekiya S, Büscher D, Izpisúa Belmonte JC, Taniguchi H (May 2008). "Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19ARF expression". Development. 135 (9): 1589–95. doi:10.1242/dev.016634. PMID 18356246.
- Begum S, Papaioannou VE (December 2011). "Dynamic expression of Tbx2 and Tbx3 in developing mouse pancreas". Gene Expression Patterns. 11 (8): 476–83. doi:10.1016/j.gep.2011.08.003. PMC 3200443. PMID 21867776.
- Volckaert T, De Langhe SP (March 2015). "Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development". Developmental Dynamics. 244 (3): 342–66. doi:10.1002/dvdy.24234. PMC 4344844. PMID 25470458.
- Washkowitz AJ, Gavrilov S, Begum S, Papaioannou VE (2012-02-14). "Diverse functional networks of Tbx3 in development and disease". Wiley Interdisciplinary Reviews. Systems Biology and Medicine. 4 (3): 273–83. doi:10.1002/wsbm.1162. PMC 3328642. PMID 22334480.
- Rowley M, Grothey E, Couch FJ (April 2004). "The role of Tbx2 and Tbx3 in mammary development and tumorigenesis". Journal of Mammary Gland Biology and Neoplasia. 9 (2): 109–18. doi:10.1023/b:jomg.0000037156.64331.3f. PMID 15300007.
- Sheeba CJ, Logan MP (2017). "The Roles of T-Box Genes in Vertebrate Limb Development". Current Topics in Developmental Biology. Elsevier. 122: 355–381. doi:10.1016/bs.ctdb.2016.08.009. ISBN 9780128013809. PMID 28057270.
- Lüdtke TH, Rudat C, Wojahn I, Weiss AC, Kleppa MJ, Kurz J, et al. (October 2016). "Tbx2 and Tbx3 Act Downstream of Shh to Maintain Canonical Wnt Signaling during Branching Morphogenesis of the Murine Lung". Developmental Cell. 39 (2): 239–253. doi:10.1016/j.devcel.2016.08.007. PMID 27720610.
- Gilbert PM, Corbel S, Doyonnas R, Havenstrite K, Magnusson KE, Blau HM (April 2012). "A single cell bioengineering approach to elucidate mechanisms of adult stem cell self-renewal". Integrative Biology. 4 (4): 360–7. doi:10.1039/c2ib00148a. PMC 3325106. PMID 22327505.
- Lu R, Yang A, Jin Y (March 2011). "Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells". The Journal of Biological Chemistry. 286 (10): 8425–36. doi:10.1074/jbc.m110.202150. PMC 3048727. PMID 21189255.
- Russell R, Ilg M, Lin Q, Wu G, Lechel A, Bergmann W, et al. (December 2015). "A Dynamic Role of TBX3 in the Pluripotency Circuitry". Stem Cell Reports. 5 (6): 1155–1170. doi:10.1016/j.stemcr.2015.11.003. PMC 4682344. PMID 26651606.
- Han J, Yuan P, Yang H, Zhang J, Soh BS, Li P, et al. (February 2010). "Tbx3 improves the germ-line competency of induced pluripotent stem cells". Nature. 463 (7284): 1096–100. Bibcode:2010Natur.463.1096H. doi:10.1038/nature08735. PMC 2901797. PMID 20139965.
- Frank DU, Emechebe U, Thomas KR, Moon AM (2013-07-02). Dettman R (ed.). "Mouse TBX3 mutants suggest novel molecular mechanisms for Ulnar-mammary syndrome". PLOS ONE. 8 (7): e67841. Bibcode:2013PLoSO...867841F. doi:10.1371/journal.pone.0067841. PMC 3699485. PMID 23844108.
- Sardar S, Kerr A, Vaartjes D, Moltved ER, Karosiene E, Gupta R, Andersson Å (January 2019). "The oncoprotein TBX3 is controlling severity in experimental arthritis". Arthritis Research & Therapy. 21 (1): 16. doi:10.1186/s13075-018-1797-3. PMC 6329118. PMID 30630509.
- Willmer T, Cooper A, Peres J, Omar R, Prince S (July 2017). "The T-Box transcription factor 3 in development and cancer". Bioscience Trends. 11 (3): 254–266. doi:10.5582/bst.2017.01043. PMID 28579578.
- Linden H, Williams R, King J, Blair E, Kini U (December 2009). "Ulnar Mammary syndrome and TBX3: expanding the phenotype". American Journal of Medical Genetics. Part A. 149A (12): 2809–12. doi:10.1002/ajmg.a.33096. PMID 19938096.
- Meneghini V, Odent S, Platonova N, Egeo A, Merlo GR (March 2006). "Novel TBX3 mutation data in families with ulnar-mammary syndrome indicate a genotype-phenotype relationship: mutations that do not disrupt the T-domain are associated with less severe limb defects". European Journal of Medical Genetics. 49 (2): 151–8. doi:10.1016/j.ejmg.2005.04.021. PMID 16530712.
- Carlson H, Ota S, Campbell CE, Hurlin PJ (October 2001). "A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnar-mammary syndrome". Human Molecular Genetics. 10 (21): 2403–13. doi:10.1093/hmg/10.21.2403. PMID 11689487.
- Kumar P., Pavan Franklin, Sarah Emechebe, Uchenna Hu, Hao Moore, Barry Lehman, Chris Yandell, Mark Moon, Anne M. (2014-03-27). TBX3 Regulates Splicing In Vivo: A Novel Molecular Mechanism for Ulnar-Mammary Syndrome. Public Library of Science. OCLC 908304248.CS1 maint: multiple names: authors list (link)
- Julià A, Ballina J, Cañete JD, Balsa A, Tornero-Molina J, Naranjo A, et al. (August 2008). "Genome-wide association study of rheumatoid arthritis in the Spanish population: KLF12 as a risk locus for rheumatoid arthritis susceptibility". Arthritis and Rheumatism. 58 (8): 2275–86. doi:10.1002/art.23623. PMID 18668548.
- Feng X, Yao W, Zhang Z, Yuan F, Liang L, Zhou J, et al. (July 2018). "T-box Transcription Factor Tbx3 Contributes to Human Hepatocellular Carcinoma Cell Migration and Invasion by Repressing E-Cadherin Expression". Oncology Research. 26 (6): 959–966. doi:10.3727/096504017x15145624664031. PMID 29295731.
- Dong L, Dong Q, Chen Y, Li Y, Zhang B, Zhou F, et al. (2018-08-24). "Novel HDAC5-interacting motifs of Tbx3 are essential for the suppression of E-cadherin expression and for the promotion of metastasis in hepatocellular carcinoma". Signal Transduction and Targeted Therapy. 3 (1): 22. doi:10.1038/s41392-018-0025-6. PMC 6107554. PMID 30151243.
- Wang Y (April 2018). "TBX3 gene in renal carcinoma and its clinical significance". Oncology Letters. 15 (4): 4235–4240. doi:10.3892/ol.2018.7841. PMC 5835868. PMID 29541189.
- Jones PA, Baylin SB (June 2002). "The fundamental role of epigenetic events in cancer". Nature Reviews. Genetics. 3 (6): 415–28. doi:10.1038/nrg816. PMID 12042769.
- Brummelkamp TR, Kortlever RM, Lingbeek M, Trettel F, MacDonald ME, van Lohuizen M, Bernards R (February 2002). "TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence". The Journal of Biological Chemistry. 277 (8): 6567–72. doi:10.1074/jbc.m110492200. PMID 11748239.
- Li X, Ruan X, Zhang P, Yu Y, Gao M, Yuan S, et al. (May 2018). "KIP2 repression". Oncogene. 37 (21): 2773–2792. doi:10.1038/s41388-017-0090-2. PMID 29511350.
- Perkhofer L, Walter K, Costa IG, Carrasco MC, Eiseler T, Hafner S, et al. (September 2016). "Tbx3 fosters pancreatic cancer growth by increased angiogenesis and activin/nodal-dependent induction of stemness". Stem Cell Research. 17 (2): 367–378. doi:10.1016/j.scr.2016.08.007. PMID 27632063.
- Li J, Weinberg MS, Zerbini L, Prince S (November 2013). "The oncogenic TBX3 is a downstream target and mediator of the TGF-β1 signaling pathway". Molecular Biology of the Cell. 24 (22): 3569–76. doi:10.1091/mbc.e13-05-0273. PMC 3826994. PMID 24025717.
- Boyd SC, Mijatov B, Pupo GM, Tran SL, Gowrishankar K, Shaw HM, et al. (May 2013). "Oncogenic B-RAF(V600E) signaling induces the T-Box3 transcriptional repressor to repress E-cadherin and enhance melanoma cell invasion". The Journal of Investigative Dermatology. 133 (5): 1269–77. doi:10.1038/jid.2012.421. PMC 3788590. PMID 23190890.
- Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan B, et al. (March 2014). "A new PKCα/β/TBX3/E-cadherin pathway is involved in PLCε-regulated invasion and migration in human bladder cancer cells". Cellular Signalling. 26 (3): 580–93. doi:10.1016/j.cellsig.2013.11.015. PMID 24316392.
- Beukers W, Kandimalla R, Masius RG, Vermeij M, Kranse R, van Leenders GJ, Zwarthoff EC (April 2015). "Stratification based on methylation of TBX2 and TBX3 into three molecular grades predicts progression in patients with pTa-bladder cancer". Modern Pathology. 28 (4): 515–22. doi:10.1038/modpathol.2014.145. PMID 25394776.
- White-Al Habeeb NM, Ho LT, Olkhov-Mitsel E, Kron K, Pethe V, Lehman M, et al. (September 2014). "Integrated analysis of epigenomic and genomic changes by DNA methylation dependent mechanisms provides potential novel biomarkers for prostate cancer". Oncotarget. 5 (17): 7858–69. doi:10.18632/oncotarget.2313. PMC 4202166. PMID 25277202.
- Kandimalla R, van Tilborg AA, Kompier LC, Stumpel DJ, Stam RW, Bangma CH, Zwarthoff EC (June 2012). "Genome-wide analysis of CpG island methylation in bladder cancer identified TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers". European Urology. 61 (6): 1245–56. doi:10.1016/j.eururo.2012.01.011. PMID 22284968.
- Etcheverry A, Aubry M, de Tayrac M, Vauleon E, Boniface R, Guenot F, et al. (December 2010). "DNA methylation in glioblastoma: impact on gene expression and clinical outcome". BMC Genomics. 11 (1): 701. doi:10.1186/1471-2164-11-701. PMC 3018478. PMID 21156036.
- Willmer T, Cooper A, Sims D, Govender D, Prince S (February 2016). "The T-box transcription factor 3 is a promising biomarker and a key regulator of the oncogenic phenotype of a diverse range of sarcoma subtypes". Oncogenesis. 5 (2): e199. doi:10.1038/oncsis.2016.11. PMC 5154352. PMID 26900951.
External links
- TBX3+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: O15119 (T-box transcription factor TBX3) at the PDBe-KB.