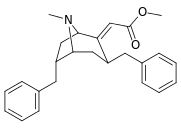
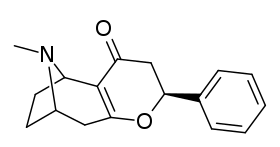
List of cocaine analogues
This is a list of cocaine analogues. A cocaine analogue is a (usually) artificial construct of a novel chemical compound from (often the starting point of natural) cocaine's molecular structure, with the result product sufficiently similar to cocaine to display similarity in, but alteration to, its chemical function. Within the scope of analogous compounds created from the structure of cocaine, so named "cocaine analogues" retain 3β-benzoyloxy or similar functionality (the term specifically used usually distinguishes from phenyltropanes, but in the broad sense generally, as a category, includes them) on a tropane skeleton, as compared to other stimulants of the kind. Many of the semi-synthetic cocaine analogues proper which have been made & studied have consisted of among the nine following classes of compounds:[lower-alpha 1]
- stereoisomers of cocaine
- 3β-phenyl ring substituted analogues
- 2β-substituted analogues
- N-modified analogues of cocaine
- 3β-carbamoyl analogues
- 3β-alkyl-3-benzyl tropanes
- 6/7-substituted cocaines
- 6-alkyl-3-benzyl tropanes
- piperidine homologues of cocaine

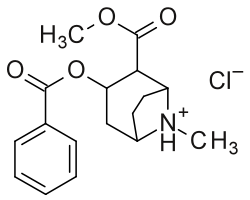
Below: Alternate two-dimensional molecular diagram of cocaine; shown specifically as a protonated, NH+, hydrochloride, and disregarding 3D stereochemistry
2′ (6′) = ortho, 3′ (5′) = meta & 4′ = para
However strict analogues of cocaine would also include such other potential combinations as phenacyltropanes & other carbon branched replacements not listed above. The term may also be loosely used to refer to drugs manufactured from cocaine or having their basis as a total synthesis of cocaine, but modified to alter their effect & QSAR. These include both intracellular sodium channel blocker anaesthetics and stimulant dopamine reuptake inhibitor ligands (such as certain, namely tropane-bridged-excised, piperidines). Additionally, researchers have supported combinatorial approaches for taking the most promising analogues currently elucidated and mixing them to the end of discovering novel & efficacious compounds to optimize their utilization for differing distinct specified purposes.[lower-alpha 2]

Although the carbmethoxy is denoted in its function as a hydrogen bond in this depiction, it has been found that it is primarily electrostatic factors which dominate binding within this space of the molecular surface area over the operative principle of hydrogen bonding.[lower-alpha 3]
Analogs sensu stricto
Cocaine Stereoisomers

| Stereoisomer | S. Singh's alphanumeric assignation |
IC50 (nM) [3H]WIN 3542 inhibition to rat striatal membranes Mean error standard ≤5% in all cases |
IUPAC nomenclature |
|---|---|---|---|
| R-cocaine (Erythroxyline) |
— | 102 | methyl(1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| R-pseudococaine (Delcaine, Depsococaine, Dextrocaine, Isococaine, Psicaine.[2]) |
172 | 15800 | methyl(1R,2S,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| R-allococaine | 173 | 6160 | methyl(1R,2R,3R,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| R-allopseudococaine | 174 | 28500 | methyl(1R,2S,3R,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate |
| S-cocaine | 175 | 15800 | methyl(1S,3R,4R,5R)-3-(benzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate |
| S-pseudococaine | 176 | 22500 | methyl(1S,3R,4S,5R)-3-(benzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate |
| S-allococaine | 177 | 9820 | methyl(1S,3S,4R,5R)-3-(benzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate |
| S-allopseudococaine | 178 | 67700 | methyl(1S,3S,4S,5R)-3-(benzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate |
Where the 2D diagrams given for the structural analogs below do not indicate stereochemistry, it should be assumed they share the conformation of R-cocaine, unless noted otherwise.
The natural isomerism of cocaine is unstable in several ways besides having a high degree of lability; for instance: the C2 carbomethoxy in its biosynthesis end-product maintains the axial position, which can undergo epimerization via saponification to obtain the former in an equatorial position.
The creation of the following analogues of cocaine have traditionally required a step which has utilized 2-CMT as an intermediate molecular product.
Benzoyl branch cleavage substitutions (excluding the exhaustive phenyl group)
| Salicylmethylecgonine[3] | Methylvanillylecgonine[4] |
 | 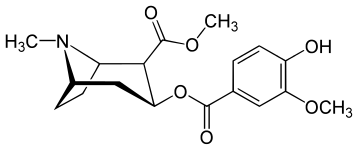 |
N.B. Fries rearrangement product of aspirin used to make salbutamol. It is relevant to the precursor here though because the migrated acetyl group can be the subject of a haloform reaction. A more direct route to vanillic acid though is just oxidation of the vanillin to a functionalized benzoic acid.
Arene benzene-ring 2′, 3′, 4′ (5′ & 6′) position (aryl) substitutions
para-substituted benzoylmethylecgonines



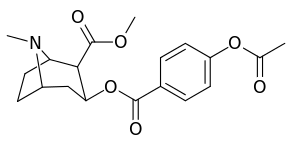

| Structure | S. Singh's alphanumeric assignation (name) |
4′=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
| (cocaine) | H | 249 ± 37 | 615 ± 120 | 2500 ± 70 | 2.5 | 10.0 | |
| non-benzoyloxy analogue comparative ligands non-tropane analogue comparative ligands | 11b (WIN 35428) (nisoxetine) (fluoxetine) | F — — | 24 ± 4 775 ± 20 5200 ± 1270 | 690 ± 14 762 ± 90 15 ± 3 | 258 ± 40 135 ± 21 963 ± 158 | 28.7 1.0 0.003 | 10.7 0.2 0.2 |
 | |||||||
| 183a | I | 2522 ± 4 | 1052 ± 23 | 18458 ± 1073 | 0.4 | 7.3 | |
| 183b | Ph | 486 ± 63 | - | - | - | - | |
| 183c | OAc | 144 ± 2 | - | - | - | - | |
| 183d | OH | 158 ± 8 | 3104 ± 148 | 601 ± 11 | 19.6 | 3.8 | |
| (4′-Fluorococaine)[5] | F | - | - | - | - | - | |
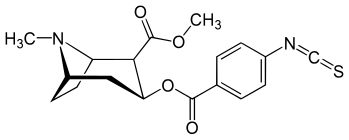
| (para-Isothiocyanatobenzoylecgonine methyl ester)[6] (p-Isococ) | NCS | - | - | - | - | - |
The MAT binding pocket analogous to the lipophilic place on cocaine-like compounds, inclusive of the benzene ring, is approximate to 9 Å in length. Which is only slightly larger than a phenyl ring by itself.[lower-alpha 6]
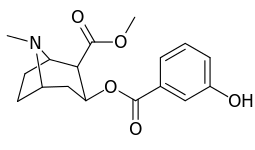
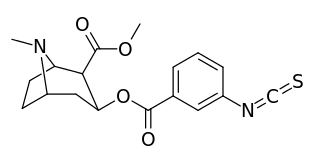
meta-substituted benzoylmethylecgonines


| Structure | S. Singh's alphanumeric assignation (name) |
3′=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
 | |||||||
| 184a | I | 325ɑ | - | - | - | - | |
| 184b | OH | 1183 ± 115 | 793 ± 33 | 3760 ± 589 | 0.7 | 3.2 | |
| 191 | OBn | - | - | - | - | - | |
| (m-Isococ) | NCS | - | - | - | - | - |
- ɑIC50 value for displacement of [3H]cocaine
ortho-substituted benzoylmethylecgonines
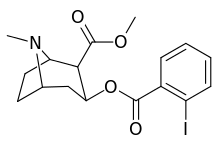
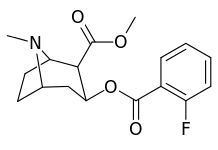
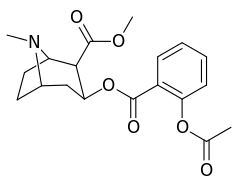
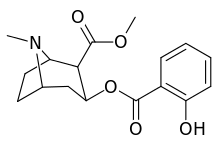
The hydroxylated 2′-OH analogue exhibited a tenfold increase in potency over cocaine.[lower-alpha 8]
| Structure | S. Singh's alphanumeric assignation (name) |
2′=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
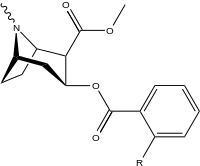 | |||||||
| 185a | I | 350ɑ | - | - | - | - | |
| 185b | F | 604 ± 67 | 1770 ± 309 | 1392 ± 173 | 2.9 | 2.3 | |
| 185c (2′-Acetoxycocaine)[7] | OAc | 70 ± 1 | 219 ± 20 | 72 ± 9 | 3.1 | 1.0 | |
| 185d (2′-Hydroxycocaine)[3] | OH | 25 ± 4 | 143 ± 21 | 48 ± 2 | 5.7 | 1.9 |
- ɑIC50 value for displacement of [3H]cocaine
manifold benzoyloxy phenyl-substitutions


Multi-substitutions (substitutions of substitutions; e.g. meta- & para-) or manifold ("many-fold") substituted analogues are analogues where more than one modification from the parent molecule takes place (having numerous intermediary constituents). These are created with often surprising structure–activity relationship results extrapolated therefrom. It is even a common case where two separate substitutions can each yield a weaker, lower affinity or even wholly non-efficacious compound respectively; but due to findings that oftentimes, when used together, such two mutually inferior changes being added in tandem to one analogue has the potential to make the resultant derivative display much greater efficacy, affinity, selectivity &/or strength than even the parent compound; which otherwise was compromised by either of those two alternations when made alone.
For an exposition & allusion to this mechanism observe that the opioid oxycodone, derived from codeine, is 1.5×—1.7× the analgesic potency of morphine (an opioid to which codeine is by comparison only 8%—12% as potent relatively, or 0.17th its strength in rats); yet oxycodone's intermediates in its synthesis from codeine are: ⅓ the potency of codeine (i.e. codeinone); 0.13 that of morphine (i.e. 14-hydroxycodeine) in rats and less in mice (to illustrate: the former even being less than the 0.17 of morphine that codeine is); with the final possible stand alone intermediate compound between codeine & oxycodone (i.e. 7,8-dihydrocodeine) being at most 150% to 200% that of codeine.[8]
| Structure | S. Singh's alphanumeric assignation (name) |
ortho-2′=R | meta-3′=R | para-4′=R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|---|---|
 | 186 | HO | H | I | 215 ± 19 | 195 ± 10 | 1021 ± 75 | 0.9 | 4.7 |
 | (Vanillylmethylecgonine)[4] | H | OCH3 | OH | - | - | - | - | - |
benzoyl phenyl-alterations


The naphthalene analogs allow for further numeric substitutions, including eight position peri substituted patterns. Many more alterations creating differing aromatic rings are possible.
| Structure | S. Singh's alphanumeric assignation (name) |
C=R | DAT
[3H]Cocaine (IC50) |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
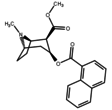 | 187 | 1-naphthalene | 742 ± 48 | - | - | - | - |
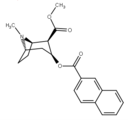 | 188 | 2-naphthalene | 327 ± 63 | - | - | - | - |
Benzoyl branch modifications
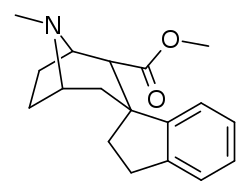
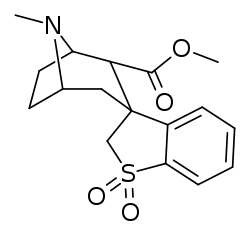
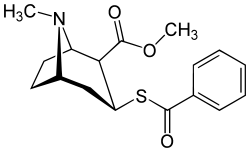
A sulfur in place of the oxygen at the benzoyl ester single bond results in a lower electronegativity than that of cocaine.
C1-tropane-ring hydrogen—substitutions

cf. hydroxytropacocaine for a natural alkaloid (lacking however, the 2-position carbmethoxy) that is a C1 substituent with a hydroxy group.
| Structure | Trivial name | R (C1 moiety) |
Ki (nM) @ DAT | Ki (nM) @ SERT | Ki (nM) @ NET | σ1 affinity Ki |
σ2 affinity Ki |
IC50 (μM) Na+ inhibition (Vertridine-Stimulated influx of sodium channels in Neocortical neurons)c |
LogP (XLogP3 algorithm, Cheng et al., 2007) |
|---|---|---|---|---|---|---|---|---|---|
| (—)-Cocaine | H | 326 ± 106 | 513 ± 143 | 358 ± 69 | 6.7 ± 0.3 μMd[13] | "significant"[14] | 6.99 ± 2.43 | 2.30 | |
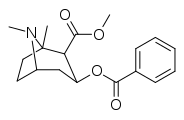 | (—)-1-methyl-cocaine | Me | 163 ± 23 | 435 ± 77 | 488 ± 101 | "unappreciable" | 1.13 μM | 16.01 ± 1.90 | 2.67 |
 | (—)-1-ethyl-cocaine | Et | 95.1 ± 17.0ɑ | 1,106 ± 112 | 598 ± 179 | — | — | — | 3.20 |
 | (—)-1-n-propyl-cocaine | n-Pr | 871 ± 205ɑ | 2,949 ± 462b | 796 ± 195 | — | — | — | 3.56 |
 | (—)-1-n-pentyl-cocaine | n-C5H11 | 1,272 ± 199b | 1,866 ± 400ɑ | 1,596 ± 21b | — | — | — | 4.64 |
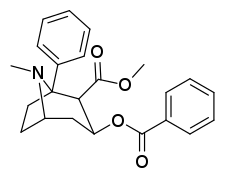 | (—)-1-phenyl-cocaine | Ph | 32.3 ± 5.7b | 974 ± 308 | 1,980 ± 99b | 524 nM | 198 nM | 0.29 ± 0.07 | 3.77 |
- ɑ, P < 0.05 compared with (—)-cocaine (one-way ANOVA followed by Dunnett's multiple comparisons test)
- b, P < 0.01 compared with (—)-cocaine (one-way ANOVA followed by Dunnett's multiple comparisons test)
- cLidocaine was found to have a value of 39.6 ± 2.4, the weakest of all tested.
- dSame reference gives 25.9 ± 2.4 μM for (+)-cocaine and 13.6 ± 1.3 μM for norcocaine. Comparably it gives 12.7 ± 1.5 μM for the sigmaergic affinity of (+)-amphetamine. Another reference gives 1.7-6.7 μM for (—)-cocaine. All values Ki.[15]
- Using same data-set as above table, the following compounds were found to compare as:
- CFT @ DAT = 39.2 ± 7.1 (n = 5)
- fluoxetine @ SERT = 27.3 ± 9.2 (n = 3)
- desipramine @ NET = 2.74 ± 0.59 (n = 3)
Cocaine analogs substituting the C1-tropane ring position, requiring sulfinimine (N-sulfinyl-imine) chemistry (before the innovation of which were untenable) which bind unlike the typical configuration at DAT (open to out) as cocaine (with its terminal D79-Y156 distance of 6.03 Å), or in the atypical (closed to out) conformation of the benztropines (3.29 Å). Though closer to the open to out: (—)-1-methyl-cocaine = 4.40 Å & (—)-1-phenyl-cocaine = 4.89 Å, and exhibiting preferential interaction with outward facing DAT conformation, they appear to have the lack of behavioral stimulation as-like the closed to out type. Despite having non-stimulant behavior profiles, they still seem to have anti-depressant behavioral profiles.[12]
The C1 phenyl analog is ten times stronger than cocaine as a dopamine reuptake pump ligand, and twenty four times stronger as a local anesthetic (voltage-dependent Na+ channel blocker), whereas the C1 methyl analog is 2.3 times less potent as a local anesthetic.[12]
2β-substitutions (including transesterification metabolite substitution cocaethylene)
.svg.png)





The consideration that large, bulky C2 substituents would alter the tropane by distorting the piperidine ring part of its skeleton sufficiently enough to impair its functionality, or that in said event such would hamper binding, in particular at the 8-aza end to ease steric strain going toward its place from the 2-position,[lower-alpha 12] appear to in many cases be unfounded.[lower-alpha 13] (examples shown in table of images below)
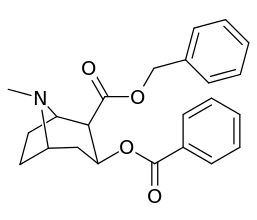 | 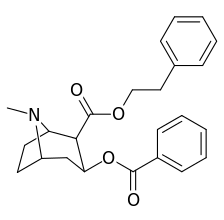 |  | 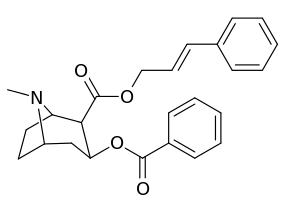 |



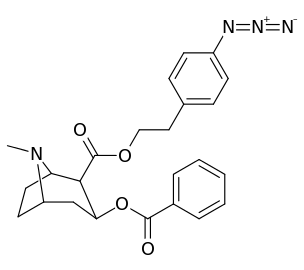
||(greater)>@ DAT, (lesser)<@ SERT, (much less)<<@ NET
 | 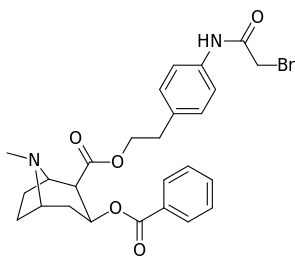 | 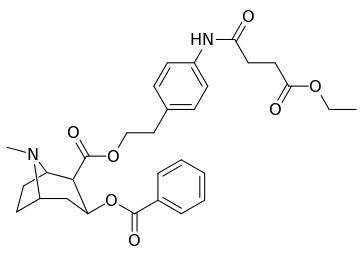 |  |




Compound 197b displayed a 1,131-fold increased selectivity in affinity over the serotonin transporter, with only slight reductions in potency for the dopamine & norepinephrine transporters.[lower-alpha 14] Whereas 197c had a 469× increase at SERT, with greater affinity for DAT than cocaine & was approximately equipotent to NET.[lower-alpha 15] 197b was 137×, and 196c 27× less potent at binding to the serotonin transporter, but both had a NET / DAT ratio that made for a better dopaminergic than cocaine.[lower-alpha 16]
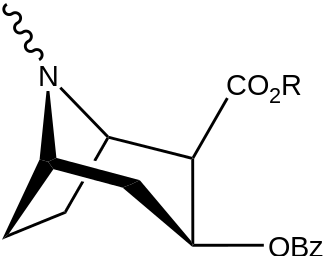
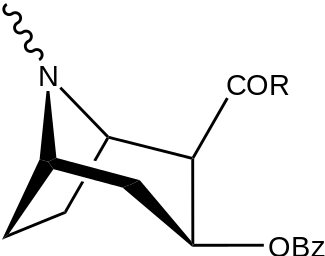

| Structure | S. Singh's alphanumeric assignation (name) |
R | DAT
[3H]WIN 35428 |
5-HTT
[3H]Paroxetine |
NET
[3H]Nisoxetine |
Selectivity
5-HTT/DAT |
Selectivity
NET/DAT |
|---|---|---|---|---|---|---|---|
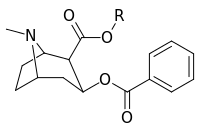 | |||||||
| (Cocaine) | Me | 89 ± 4.8 | 1045 ± 89 | 3298 ± 293 | 11.7 | 37.0 | |
| 196a (Cocaethylene) | Et | 195 ± 45 | 5801 ± 493 | 10000 ± 751 | 29.7 | 51.3 | |
| 196b | n-Pr | 196 ± 46 | 4517 ± 430 | 6124 ± 262 | 23.3 | 31.2 | |
| 196c | i-Pr | 219 ± 48 | 25224 ± 1498 | 30384 ± 1685 | 115 | 139 | |
| 196d | Ph | 112 ± 31 | 33666 ± 3330 | 31024 ± 1909 | 300 | 277 | |
| 196e | Bn | 257 ± 14 | 302 ± 23 | 20794 ± 950 | 1.2 | 80.9 | |
| 196f | β-phenethyl | 181 ± 10 | 615 ± 52 | 19944 ± 1026 | 3.4 | 110 | |
| 196g | γ-phenylpropyl | 147 ± 19 | 374 ± 15 | 4893 ± 344 | 2.5 | 33.3 | |
| 196h | cinnamyl | 371 ± 15 | 368 ± 6.3 | 68931 ± 3476 | 1.0 | 186 | |
| 196i | p-NO2-β-phenethyl | 601 ± 28 | - | - | - | - | |
| 196j | p-Cl-β-phenethyl | 271 ± 12 | - | - | - | - | |
| 196k | p-NH2-β-phenethyl | 72 ± 7 | - | - | - | - | |
| 196l | p-NCS-β-phenethyl | 196 ± 14 | - | - | - | - | |
| 196m | p-azido-β-phenethyl | 227 ± 19 | - | - | - | - | |
| 196n | (p-NHCOCH2Br)β-phenethyl | 61 ± 6 | - | - | - | - | |
| 196o | (p-NHCO(CH2)2CO2Et)β-phenethyl | 86 ± 4 | - | - | - | - | |
 | 197a | NH2 | 753 ± 41.3 | 13725 ± 1256 | 3981 ± 229 | 18.2 | 5.3 |
| 197b | -NMe2 | 127 ± 6.36 | 143713 ± 8854 | 7329 ± 158 | 1131 | 57.7 | |
| 197c | -N(OMe)Me | 60 ± 6.4 | 28162 ± 2565 | 3935 ± 266 | 469 | 65.6 | |
| 197d | -NHMe | 2424 ± 118 | 44798 ± 2105 | 4213 ± 206 | 18.5 | 1.7 | |
| 197e (Benzoylecgonine) | -OH | 195000 | - | - | - | - | |
 | 197f | HOCH2- | 561 ± 149 | - | - | - | - |
| 197g (Tropacocaine) | H | 5180 ± 1160 | - | - | - | - |
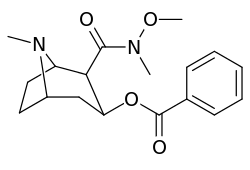
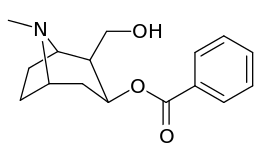
Bioisostere 2-position carbmethoxy-ester functional replacements






Benzoylecgonine, i.e. compound 197e, (differing from its cocaine parent only by de-methylation of the C2 carbmethoxy to that of a carboxy) has an extreme loss in potency (its approximate affinity being 195,000 nM) as displayed by in vitro methodologies for determining binding efficacy (wherein BBB penetration does not factor-in on the matter in the manner as in vivo studies) and is posited to be due possibly to zwitterion formation.[lower-alpha 18]

| Structure | S. Singh's alphanumeric assignation (name) |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| (Cocaine) | (H) | 580 ± 70 | 570 ± 180 | 1.0 | |
 | |||||
| 198a | H | 520 ± 40 | 260 ± 70 | 0.5 | |
| 198b | CO2Et (5′-carboethoxy-) | 120 ± 10 | 290 ± 40 | 2.4 | |
| 198c | BOC | 2230 ± 220 | 1820 ± 810 | 0.8 | |
| 198d | Ph | 2000 ± 640 | 2920 ± 1620 | 1.5 | |
| 198e | CH=CHCO2Me | 3600 ± 400 | 3590 ± 1180 | 1.0 |
methyl)_cocaine_analogue.png)

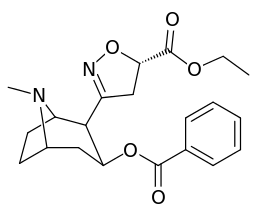
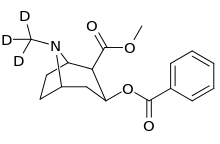
| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| 199a | β(or R)CO2Et | 710 ± 150 | 1060 ± 340 | 1.5 | |
| 199b | α(or S)CO2Et | 5830 ± 630 | 8460 ± 620 | 1.4 |
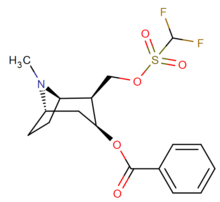
| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| 200 | 880 ± 350 | 400 ± 140 | 0.4 |
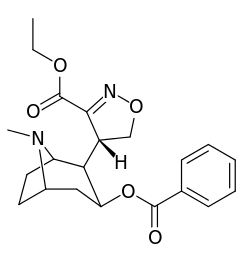
Vinylogous 2β-position carbmethoxy-ester functional replacements

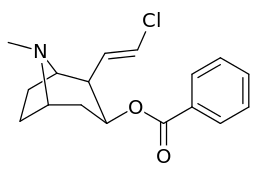
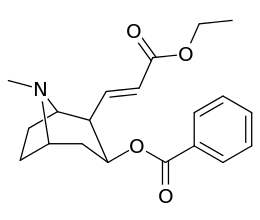

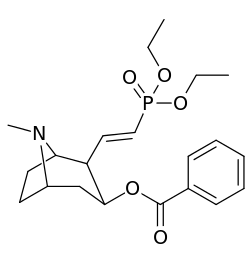
201b & 201c show significant increased potency over cocaine; whereas 201a, 201d & 201e are considerably less so. This infers the hydrogen bond acceptor at the 2β position to not necessarily be of exclusive import in creation of higher binding analogues of cocaine.

| Structure | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
 | |||||
| 201a | H | 1730 ± 550 | 1120 ± 390 | 0.6 | |
| 201b | Cl | 222 ± 49 | 368 ± 190 | 1.6 | |
| 201c | CO2Et | 50 ± 10 | 130 ± 10 | 2.6 | |
| 201d | CH=CHCO2Et | 1220 ± 100 | 870 ± 50 | 0.7 | |
| 201e | PO(OEt)2 | 4850 ± 470 | 5500 ± 70 | 1.1 |
N-modifications
.svg.png)







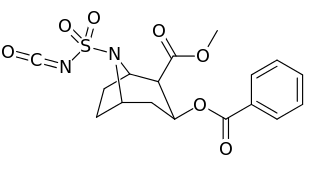

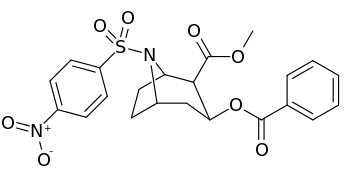
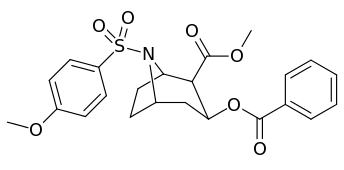
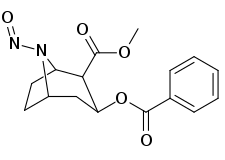



-6-methyl-6-azabicyclo(3.2.1)octan-3-yl_2-phenylbenzoate.svg.png)
| Compound | S. Singh's alphanumeric assignation (name) |
N8-R | [3H]Mazindol binding |
[3H]DA uptake |
Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
 | 217 (Cocaine methiodide) | - | 10700 ± 1530ɑ | - | - |
 | (Cocaine) | CH3 | 280 ± 60 102ɑ | 320 ± 10 | 1.1 |
| 218 (Norcocaine) | H | 303 ± 59ɑ | - | - | |
| 219a | Bn | 668 ± 67ɑ | - | - | |
| 219b | Ac | 3370 ± 1080ɑ | - | - | |
| 219c | CH2CH2OH | 700 ± 100 | 1600 ± 200 | 2.3 | |
| 219d | CH2CO2CH3 | 480 ± 40 | 1600 ± 100 | 3.3 | |
| 219e | CH2CO2H | 380 ± 20 | 2100 ± 400 | 5.5 | |
| 220a | SO2CH3 (Ms) | 1290 ± 80 | 1970 ± 70 | 1.5 | |
| 220b | SO2CF3 (Tf) | 330 ± 30 | 760 ± 20 | 2.3 | |
| 220c | SO2NCO | 120 ± 10 | 160 ± 10 | 1.3 | |
| 220d | SO2Ph | 20800 ± 3500 | 61000 | 2.9 | |
| 220e | SO2C6H4-4-NO2 (nosyl) | 5720 ± 1140 | 18800 ± 90 | 3.3 | |
| 220f | SO2C6H4-4-OCH3 | 6820 ± 580 | 16400 ± 1400 | 2.4 | |
| 221a | NO | 99500 ± 12300 | 231700 ± 39500 | 2.3 | |
| 221b | NO2 | 7500 ± 900 | 21200 ± 600 | 2.8 | |
| 221c | NHCOCH3 | >1000000 | >1000000 | - | |
| 221d | NH2 | - | - | - |
- ɑIC50 (nM) for displacement of [3H]WIN 35428
Bridged (N-constrained/tethered) tropane-fused cocaine analogues
8 to 2 position tropane bridge

See N-front & back bridged phenyltropanes.
| Compound | S. Singh's alphanumeric assignation |
R | [3H]Mazindol | [3H]DA | Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
 | 222 | 44900 ± 6200 | 115000 ± 15700 | 2.6 |
Back-bridged cocaine analogues are considered more akin to untethered cocaine analogs & phenyltropane derivatives (where the nitrogen lone pair is not fixed or constrained) and better mimics their affinities. This is due to when the eighth carbon tropane position is freely rotatable and unbound it preferably occupies the axial position as defining its least energy & most unhindered state. In front-bridged analogs the nitrogen lone pairings rigid fixity makes it reside in an equatorial placing for the piperidine ring-part of the tropane nucleus, pointing to the two-carbon & three methylene unit bridgehead; giving the attested front-bridged cocaine analogues preference for SERT over DAT.[lower-alpha 25]
Tricyclic cocaine analogues
Tethering the nitrogen 8 tropane position one position further (beyond 2β and crossed-over it / leaving it open as hydrogen and thus possible of having additional unconstrained substitutions there) and linking all the way across to the 3β aryl, replacing it; yields an expansive front-bridged structure to create a structurally tricyclic series of cocaine analogues.
8 to 3 position


1st structure (di-chloro benzene, 2β-CH2OCOMe) SERT = 1.6, DAT = 1870, NET = 638
2nd structure (para-bromo, meta-chloro, 2β-CO2Me) SERT = 2.3, DAT = 5420, NET = 459
3rd structure (para-iodo, meta-chloro, 2β-CH2OCOPh) SERT = 0.06, DAT/NET both = >10K
| Compound | X | Y | R | SERT Ki (nM) | DAT Ki (nM) | NET Ki (nM) |
| 1 | Cl | Cl | CH2OCOMe | 1.6 | 1870 | 638 |
| 2 | Br | Cl | CO2Me | 2.3 | 5420 | 459 |
| 3 | I | Cl | CH2OCOPh | 0.06 | >10K | >10K |
Azabornane tropane ring contraction
Alterations shortening the tropane ring system while including the benzoyloxy length at the C3 have been made, contrasting the azabornane phenyltropanes;[17] likely remedying the shallow penetration (for good efficacy) of the latter.
5-benzoatic (left, below) & 6-benzoatic (right, below)
-2-methyl-2-azabicyclo(2.2.1)heptan-5-yl_benzoate.svg.png)
-2-methyl-2-azabicyclo(2.2.1)heptan-6-yl_benzoate.svg.png)

Comparison of tropane ring versus the norbornane in overlay, with contrast of benzoyl branch in its configuration of how it lays extended out from body of either main ring type (shown twice in different colors, for visibility, above)
6/7 tropane position methoxycocaine & methoxypseudococaine analogues



-4-chloro-8-methyl-3-(phenylsulfanyl)-8-azabicyclo(3.2.1)oct-2-ene-2-carboxylate.svg.png)
| Compound | S. Singh's alphanumeric assignation (name) |
X | Ki (nM) [3H]Mazindol binding |
Ki (nM) [3H]DA uptake |
Selectivity
Uptake/Binding |
|---|---|---|---|---|---|
| (Cocaine) | 280 ± 60 | 320 ± 10 | 1.1 | ||
| (Pseudococaine) | 10400 ± 300 | 13800 ± 1500 | 1.3 | ||
| 225a | 2β, 6β-OCH3 | 98000 ± 12000 | 68000 ± 5000 | 0.7 | |
| 225b | 2α, 6β-OCH3 | 190000 ± 11000 | 510000 ± 110000 | 2.7 | |
| 225c | 2β, 7β-OCH3 | 4200 ± 100 | 6100 ± 200 | 1.4 | |
| 225d | 2α, 7β-OCH3 | 45000 ± 5000 | 110000 ± 4000 | 2.4 | |
| 225e | 2α, 7α-OCH3 | 54000 ± 3000 | 200000 ± 70000 | 3.7 |
3β-position 2′—(6′) & 2β-substitution combination analogues


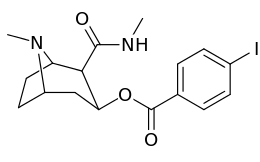

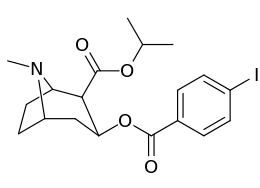

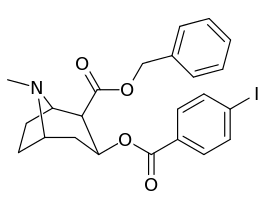
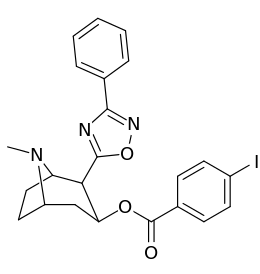


| Compound | S. Singh's alphanumeric assignation |
2β-R | C2′-R | IC50 (nM) (displacement of [3H]WIN 35428) |
|---|---|---|---|---|
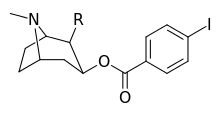 | ||||
| 211a | CO2OH | H | 6214 ± 1269 | |
| 211b | CH2OCOCH3 | H | 2995 ± 223 | |
| 211c | CONHCH3 | H | >100000 | |
| 211d | CO2Et | H | 2031 ± 190 | |
| 211e | CO2-i-Pr | H | 1377 ± 10 | |
| 211f | CO2Ph | H | 2019 ± 253 | |
| 211g | CO2CH2Ph | H | 4602 ± 325 | |
| 211h | 3-phenyl-1,2,4-oxadiazole | H | 3459 ± 60 | |
| 211i | CH=CH2 | H | 2165 ± 253 | |
| 211j | CH2CH3 | H | 2692 ± 486 | |
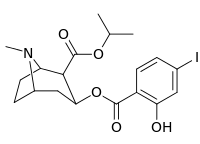 | 212 | CO2-i-Pr | HO | 663 ± 70 4507 ± 13ɑ 34838 ± 796b |
- ɑFor displacement of [3H]paroxetine (5-HTT & NET)
- bFor displacement of [3H]nisoxetine (5-HTT & NET)
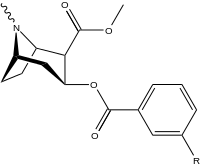
3β-Carbamoyl analogues

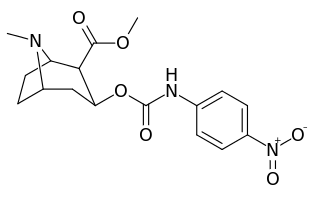



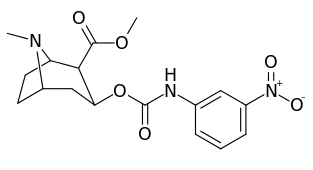



| Compound | S. Singh's alphanumeric assignation (name) |
X | IC50 (nM) inhibition of [3H]Cocaine binding (Rat Striatal Tissue) |
IC50 (nM) inhibition of [3H]DA uptake (Rat Striatal Tissue) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | (H) | 70 ± 10 | 210 ± 70 | 3.0 | |
 | |||||
| 223a | H | 5600 ± 700 | 52600 ± 3000 | 9.4 | |
| 223b | 4-NO2 | 1090 ± 250 | 5700 ± 1200 | 5.2 | |
| 223c | 4-NH2 | 63300 ± 12200 | >100000 | - | |
| 223d | 4-N3 | 1000 ± 240 | 1180 ± 360 | 1.2 | |
| 223e | 4-NCS | 260 ± 60 | 490 ± 80 | 1.9 | |
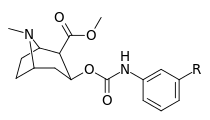 | |||||
| 223f | 3-NO2 | 37 ± 10 | 178 ± 23 | 4.8 | |
| 223g | 3-NH2 | 2070 ± 340 | 23100 ± 900 | 11.1 | |
| 223h | 3-N3 | 630 ± 150 | 3900 ± 1590 | 6.2 | |
| 223i | 3-NCS | 960 ± 210 | 4900 ± 420 | 5.1 |
Phenyl 3-position linkage substitutions
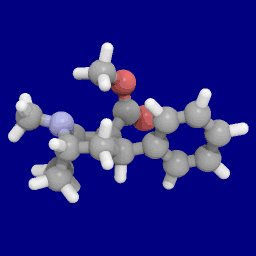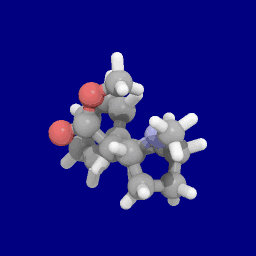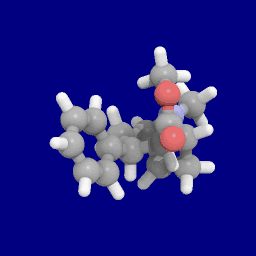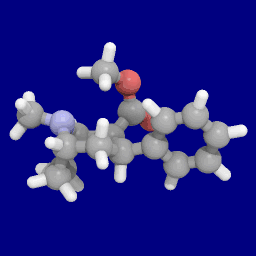

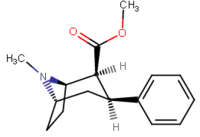
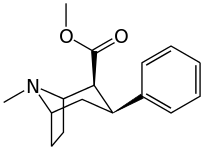
See: List of phenyltropanes (Many phenyltropanes are derived from cocaine metabolites, such as methylecgonidine, as precursors. Whereas fully synthetic methods have been devised from the starting material of vinylcarbenoids & pyrroles.)[22]
The difference in the length of the benzoyloxy and the phenyl linkage contrasted between cocaine and phenyltropanes makes for a shorter distance between the centroid of the aromatic benzene and the bridge nitrogen of the tropane in the latter PTs. This distance being on a scale of 5.6 Å for phenyltropanes and 7.7 Å for cocaine or analogs with the benzoyloxy intact.[lower-alpha 29] This may account for PTs increased behavioral stimulation profile over cocaine.[lower-alpha 30] Differences in binding potency have also been explained considering solvation effects; cocaine containing 2β,3β-ester groups being calculated as more solvated than the WIN-type compounds (i.e. troparil). Higher pKɑs of the tropane nitrogen (8.65 for cocaine, 9.55 for troparil & 11.95 for vinyl analogue 43a), decreased aqueous solvation & decreased conformational flexibility added to increased binding affinity.[lower-alpha 31]
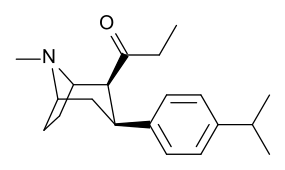
Despite the observation of increased stimulation, phenyltropanes lack the local anesthetic sodium channel blocking effect that the benzoyloxy imparts to cocaine. Beside topical affect, this gives cocaine an affinity for binding to sites on the dopamine and serotonin sodium dependent transport areas that are distinct & specific to MAT in contrast to the general sodium channels; creating a separate mechanism of relational affinity to the transporters in addition to its inhibition of the reuptake for those transporters; this is unique to the local anesthetic value in cocaine & analogues with a similar substitute for the benzoyloxy that leaves the sodium channel blockage ability intact. Rendering such compounds as different functionally in their relation to MAT contrasted to phenyltropane analogues which have the local anesthetic bridge removed.[23] (Requiring some of the sodium ions to be pumped from the axon via Na+/K+-ATPase). In addition, it even has been postulated that a crucial role regarding the electron energy imparted via voltage sensitization (and thus action potential blockage with a molecule capable of intersecting its specific channel, in the case of cocaine a sodium channel, that potentially serves in re-quantifying its charge) upon a receptor binding site may attenuate the mediating influence of the inhibitory regulation that autoreceptors play by their slowing neurotransmitter release when an efflux is created through an instance of agonism by a compound; allowing said efflux to be continued without the body's attempt to maintain homeostasis enacting in as readily responsive a manner to its conformational change.[24]
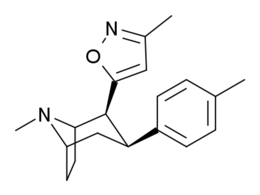

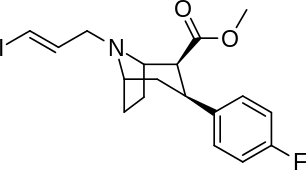
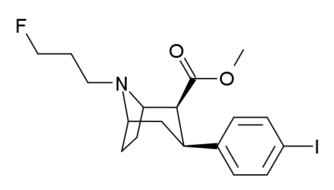
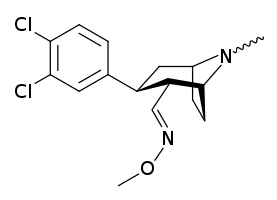
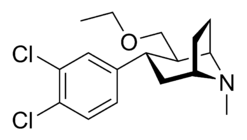
3β-Alkylphenyltropane & 3β-Alkenyl analogues
The compound 224e, the 3β-styrene analogue, had the highest potency in its group. While 224b & 224c showed the most selectivity, with 224b having a ten-fold greater potency for the dopamine transporter than cocaine.[lower-alpha 32]
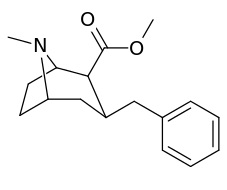
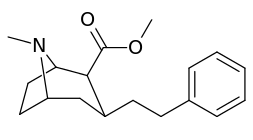
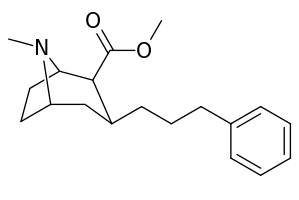

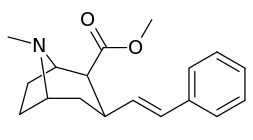
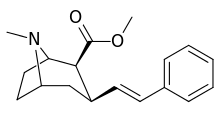
(i.e. compound "224e")
| Compound | S. Singh's alphanumeric assignation (name) |
n | IC50 (nM) [3H]Cocaine binding |
IC50 (nM) [3H]DA uptake |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | 101 ± 26 | 209 ± 20 | 2.1 | ||
 | |||||
| 224a | 1 | 885 ± 18 | 1020 ± 52 | 1.1 | |
| 224b | 2 | 9.9 ± 0.33 | 70.5 ± 1.0 | 7.1 | |
| 224c | 3 | 344 ± 12 | 2680 ± 190 | 7.8 | |
| 224d | 71.6 ± 0.7 | 138 ± 9 | 1.9 | ||
| 224e | 2.10 ± 0.04 | 5.88 ± 0.09 | 2.8 |
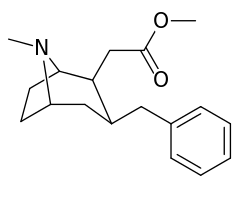
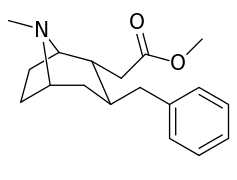
6-Alkyl-3-benzyltropane analogues
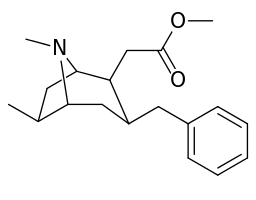




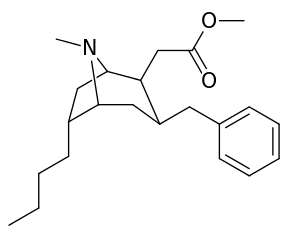

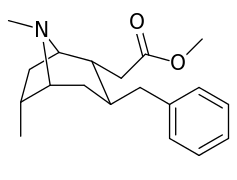





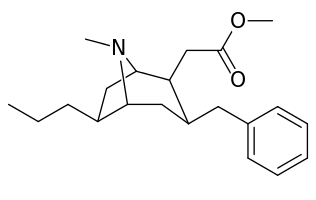





| Compound | S. Singh's alphanumeric assignation (name/WIN number) |
R | Ki (nM) [3H]WIN 35428 binding |
IC50 (nM) [3H]DA uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|
| (Cocaine) | 32 ± 5 338 ± 221 | 405 ± 91 405 ± 91 | 12.6 1.2 | ||
| 11a (WIN 35065-2) | 33 ± 17 314 ± 222 | 373 ± 10 | 11.3 | ||
| (−)-229a | H | 33 ± 5 | 161 ± 100 | 4.9 | |
| 229a | H | 91 ± 10 | 94 ± 26 | 1.0 | |
| 229b | Me | 211 ± 23 | - | - | |
| 229c | Et | 307 ± 28 | - | - | |
| 229d | n-Pr | 4180 ± 418 | - | - | |
| 229e | n-Bu | 8580 ± 249 | - | - | |
| 229f | Bn | 3080 ± 277 | - | - | |
| (+)-230a | H | 60 ± 6 | 208 ± 63 | 3.5 | |
| 230a | H | 108 ± 14 | 457 ± 104 | 4.2 | |
| 230b | Me | 561 ± 64 | - | - | |
| 230c | Et | 1150 ± 135 | - | - | |
| 230d | n-Pr | 7240 ± 376 | - | - | |
| 230e | n-Bu | 19700 ± 350 | - | - | |
| 230f | Bn | 7590 ± 53 | - | - | |
| 231b | Me | 57 ± 5 | 107 ± 36 | 1.9 | |
| 231c | Et | 3110 ± 187 | - | - | |
| 231d | n-Pr | 5850 ± 702 | - | - | |
| 231f | Bn | 1560 ± 63 | - | - | |
| 232b | Me | 294 ± 29 | 532 ± 136 | 1.8 | |
| 232c | Et | 6210 ± 435 | - | - | |
| 232d | n-Pr | 57300 ± 3440 | - | - | |
| 232f | Bn | 3080 ± 277 | - | - | |
| 241 | Bn | 4830 ± 434 | - | - |
| Sub-category (S. Singh compound #) |
a R=H |
b R=Me |
c R=Et |
d R=n-Pr |
e R=n-Bu |
f R=Bn |
|---|---|---|---|---|---|---|
| 6α-isomers: 237a—f | ||||||
 | 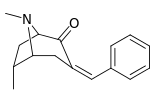 | 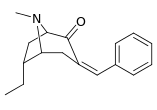 |  |  |  | |
| 6β-isomers (exo): 238a—f | ||||||
 |  |  |  |  |
| |
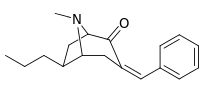
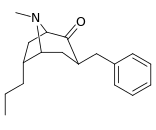
| 3β-benzyl derivatives: 239a—f | ||||||
 |  |  |  | 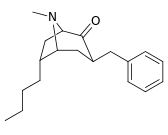 | 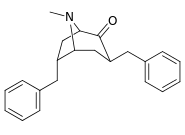 | |
| intermediate alkylidene esters: 240a—f | ||||||
 |  |  | 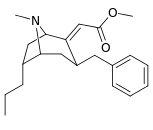 | 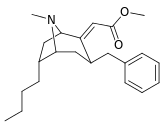 |  | |
N.B. that 237a and 238a are the same compound as both are the parent for either series with a hydrogen saturated in their respective substitution place.
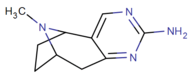
Direct 2,3-pyrimidino fused
below: Chalcostrobamine

cf. strobamine (at right) for a more efficacious compound as like the below.
| Structure | alphanumeric assignation |
R1 | R2 | hDAT IC50 (nM) |
hSERT IC50 (nM) |
hNET IC50 (nM) |
|---|---|---|---|---|---|---|
 | ||||||
| (—)-3a | H | C6H5 | 58,300 (20,200) | 6140 (3350) | NA | |
| (+)-3a | H | C6H5 | 48,700 (20,100) | 6030 (3400) | NA | |
 | ||||||
| (—)-3b | H | NH2 | NA | NA | NA | |
| (+)-3b | H | NH2 | NA | NA | NA | |
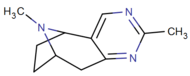 | ||||||
| (—)-3c | H | CH3 | NA | NA | NA | |
| (+)-3c | H | CH3 | NA | NA | NA | |
 | ||||||
| (—)-3d | H | H | NA | NA | NA | |
| (+)-3d | H | H | NA | NA | NA | |
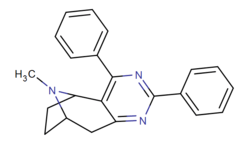 | (+/—)-3e | C6H5 | C6H5 | 30,000 (11,200) | 3650 (1700) | NA |
- "NA" = "no affinity", e.g. unquantifiable.
Direct di-hetero-benzene (pyrimidino) 2,3-fused and thus rigidified cocaine analogs.[27]
Piperidine cocaine-homologues
-8-methyl-3-(tricyclo(9.4.0.0%C2%B3%2C%E2%81%B8)pentadeca-1(11)%2C3%2C5%2C7%2C12%2C14-hexaene-2-carbonyloxy)-8-azabicyclo(3.2.1)octane-2-carboxylate.svg.png)
cf. phenyltropane piperidine-homologues for compounds with a more optimized conformation that yield higher affinities when binding to MAT.

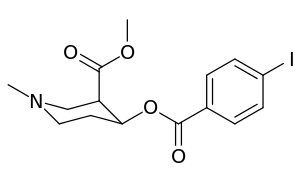
| Compound | S. Singh's alphanumeric assignation (name) |
2β-R | IC50 (nM) |
|---|---|---|---|
| (Cocaine) | CO2CH3 (i.e. CO2Me) | 249 ± 37 | |
| 183a | CO2CH3 | 2522 ± 4 | |
| 242 | H | 11589 ± 4 | |
| 243 | CO2CH3 | 8064 ± 4 |
Cocaine hapten analogues
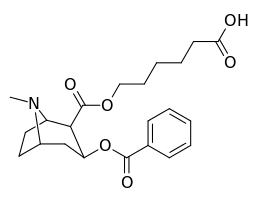
| Compound | S. Singh's alphanumeric assignation (name) |
2β-R |
|---|---|---|
 | 394 (GNC)ɑ | CO2(CH2)5CO2H |
 | 395 (Succinyl Norcocaine)[29] | CO2CH3 |
 | GNEb[30] including carrier proteins: GNE-FLiC GNE-KLH GNE-BSA | |
 | 396 | CONH(CH2)5CO2H |
- ɑ6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carbonyloxy-hexanoic acid
- b6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carboxamido-hexanoic acid
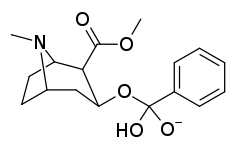
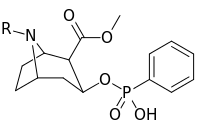
| Compound | S. Singh's alphanumeric assignation (name) |
R |
|---|---|---|
 | ||
| 401a | CH3 | |
| 401b | (CH2)5CO2H | |
| 401c | CH2CO2H | |
| 401d | COCH2CH2CO2H | |
| 401e | H | |
| 401f | CH2CH2Br | |
| 385g | (CH2)2NHCO(CH2)2CONH2 | |
 | ||
| 402a | O(CH2)4NHCO(CH2)2CO2...2,3-dihydro-1H-isoindole-1,3-dione | |
| 402b | OH | |
| 402c | O(CH2)2...1,4-xylene...NH2 | |
| 402d | NH(CH2)5CO2H | |
| 402e | O(CH2)4NHCO(CH2)2CONH2 | |
 | ||
| 403a | NH2 | |
| 403b | NHCOCH2Br | |
| 403c | NHCO(CH2)3CO2H | |
| 403d | (CH2)3NHCO(CH2)2CONH2 |
Cocaine haptens that create catalytic anti-bodies require transitional states as affected in vivo.[31][32]
| Compound | Name |
|---|---|
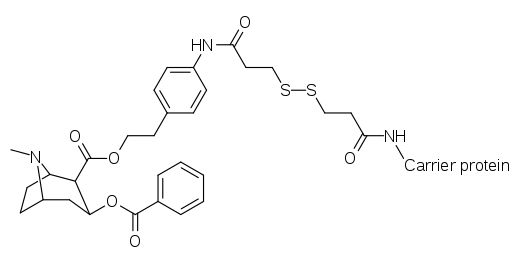 | |
| K1-KLH/BSA[34] | |
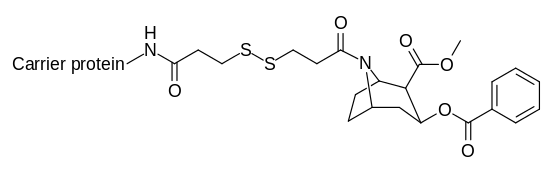 | |
| K2-KLH/BSA |
Structural/Functional intermediate analogues
Piperidine Analogues
- JZ-IV-10 (a "Modafinil hybrid" with nocaine.[35] cf. List of modafinil analogues)
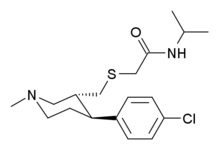
-CPCA.svg.png)
A somewhat recent occurrence among tentative modern folklore which has traversed the circling of rumors mostly confined to the likes of universities and popular culture trivia has been that cocaine is one element, or molecule increment of weight or charge etc., away from the molecular structure of sugar.[36] Though such a statement is false as a general pretense, there is a dextrose based super-structure that has a vaguely similar overlay with cocaine which is "benzoyl-beta-D-glucoside."
Benztropine (3α-Diphenylmethoxy Tropane) Analogs
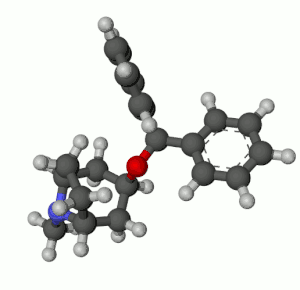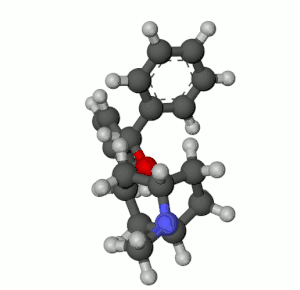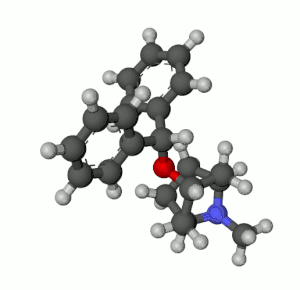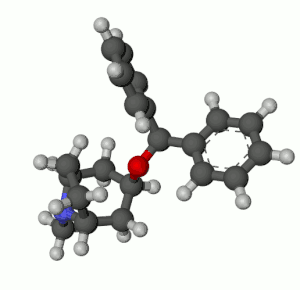
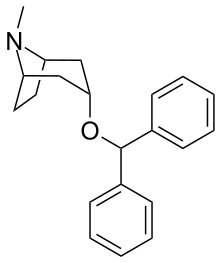
- Benzatropine (BZT)[37]
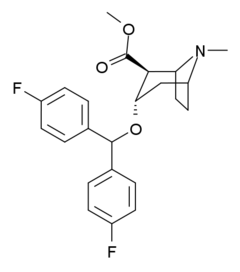
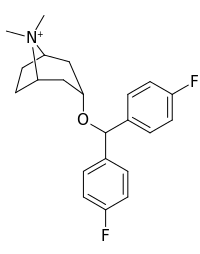
- Difluoropine (O-620), More selective as a DARI than cocaine. Also an anticholinergic & antihistamine.
- AHN 1-055 Same structure as for benztropine but 4′,4′-bisfluorinated.
- GA 103 N-phenylpropyl bis-4-fluorobenztropine.
- JHW 007[38] N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane.
Unlike cocaine & phenyltropanes, the benztropines & GBR compounds (and, as an exception to the cocaine pharmacophore itself, allotropacocaine) among others are considered "atypical" DAT re-uptake pump ligands because they stabilize the dopamine transporter in an inward-facing or closed-to-out conformation, this contrasts what is considered "cocaine-like" affinity to DAT; which would instead keep DAT stable in an open-to-out conformation. This means the binding of many dopamine reuptake inhibitors is atypical of cocaine's method of binding to DAT and significantly diverges from it.[39]
"Difluoropine" is not a phenyltropane but actually belongs to the benzatropine family of DRIs. Not to be confused for the "diaryl"-phenyltropanes.
In certain respects these are important because they share SAR overlap with GBR 12909 and related analogs.
SARs have shown that 4′,4′-difluorination is an excellent way to boost DAT activity of benztropine, and gives excellent selectivity over the SERT and the NET.[40][41]
Furthermore, replacing the N-Me with, e.g. n-phenylpropyl helps to bring muscarinic activity down to something that is the same as DRI affinity.[40]
This is remarkable considering unmodified (native) benztropine is 60 times more active as an anticholinergic than as a dopaminergic.[40]
M1 receptor considerations aside, analogues of this benztropine class still won't substitute for cocaine, and have no propensity to elevate locomotor activity.
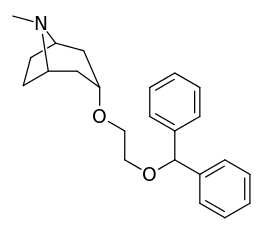
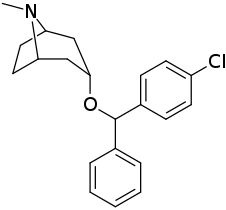



| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | Ki (nM) [3H]WIN 35428 binding |
IC50 (nM) [3H]DA uptake |
Selectivity
uptake/binding |
|---|---|---|---|---|---|---|
| (Cocaine) | 388 ± 47 | - | - | |||
| (GBR 12909) | 11.6 ± 31 | - | - | |||
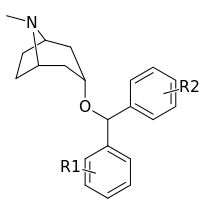 | ||||||
| (Benztropine) | H | H | 118 ± 9 | 403 ± 115 | 3.4 | |
| 249a | 4′-F | H | 32.2 ± 10 | 48 | 1.5 | |
| 249b (AHN 1-055) | 4′-F | 4′-F | 11.8 ± 1 | 71 | 6.0 | |
| 249c | 3′,4′-di-F | H | 27.9 ± 11 | 181 ± 45.7 | 6.5 | |
| 249d | 4′-Cl | H | 30.0 ± 12 | 115 | 3.8 | |
| 249e | 4′-Cl | 4′-Cl | 20.0 ± 14 | 75 | 3.8 | |
| 249f | 3′,4′-di-Cl | H | 21.1 ± 19 | 47 | 2.2 | |
| 249g | 3′,4′-di-Cl | F | 18.9 ± 14 | 24 | 1.3 | |
| 249h | 4′-Br | H | 37.9 ± 7 | 29 | 0.8 | |
| 249i | 4′-Br | 4′-Br | 91.6 | 34 | 0.4 | |
| 249j | 4′-NO2 | H | 197 ± 8 | 219 | 1.1 | |
| 249k | 4′-CN | H | 196 ± 9 | 222 | 1.1 | |
| 249l | 4′-CF3 | H | 635 ± 10 | 2155 | 3.4 | |
| 249m | 4′-OH | H | 297 ± 13 | 677 | 2.3 | |
| 249n | 4′-OMe | H | 78.4 ± 8 | 468 | 6.0 | |
| 249o | 4′-OMe | 4′-OMe | 2000 ± 7 | 2876 | 1.4 | |
| 249p | 4′-Me | H | 187 ± 5 | 512 | 2.7 | |
| 249q | 4′-Me | 4′-Me | 420 ± 7 | 2536 | 6.0 | |
| 249r | 4′-Et | H | 520 ± 8 | 984 | 1.9 | |
| 249s | 4′-t-Bu | H | 1918 | 4456 | 2.3 | |
| 250a | 3′-F | H | 68.5 ± 12 | 250 ± 64.7 | 3.6 | |
| 250b | 3′-F | 3′-F | 47.4 ± 1 | 407 ± 63.9 | 8.6 | |
| 250c | 3′-Cl | H | 21.6 ± 7 | 228 ± 77.1 | 10.5 | |
| 250d | 3′-CF3 | H | 187 ± 5 | 457 ± 72.0 | 2.4 | |
| 251a | 2′-F | H | 50.0 ± 12 | 140 ± 17.2 | 2.8 | |
| 251b | 2′-Cl | H | 228 ± 9 | 997 ± 109 | 4.4 | |
| 251c | 2′-Me | H | 309 ± 6 | 1200 ± 1.64 | 3.9 | |
| 251d | 2′-NH2 | H | 840 ± 8 | 373 ± 117 | 0.4 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|
| (benztropine) | 312 ± 1.1 | 24100 ± 14800 | 77.2 | |||
| (WIN 35428) | 12.9 ± 1.1 | 160 ± 20 | 12.4 | |||
| R-256 | 2040 ± 283 | 1460 ± 255 | 0.7 | |||
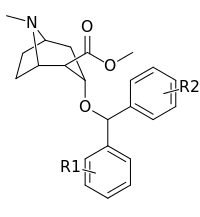 | ||||||
| S-257a | H | H | 33.5 ± 4.5 | 10100 ± 1740 | 301 | |
| S-257b | H | F | 13.2 ± 1.9 | 4930 ± 1200 | 373 | |
| S-257c (difluoropine) | F | F | 10.9 ± 1.2 | 3530 ± 1480 | 324 | |
| S-257d | H | Cl | 15.8 ± 0.95 | 5960 ± 467 | 377 | |
| S-257e | Cl | Cl | 91.4 ± 0.85 | 3360 ± 1480 | 36.8 | |
| S-257f | H | Br | 24.0 ± 4.6 | 5770 ± 493 | 240 | |
| S-257g | Br | Br | 72.0 ± 3.65 | 2430 ± 339 | 33.7 | |
| S-257h | H | I | 55.9 ± 10.3 | 9280 ± 1640 | 166 | |
| S-257i | Br | I | 389 ± 29.4 | 4930 ± 82 | 12.7 | |
| S-257j | I | I | 909 ± 79 | 8550 ± 442 | 9.4 | |
| S-257k | H | Me | 49.5 ± 6.0 | 13200 | 266 | |
| S-257l | Me | Me | 240 ± 18.4 | 9800 ± 2680 | 40.8 |

| Compound | S. Singh's alphanumeric assignation (name) |
R | n | IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|
 | ||||||
| 258a | 20.3 ± 3.5 | - | - | |||
| 258b | H | 1 | 223 ± 53 | 4970 ± 700 | 22.3 | |
| 258c | H | 3 | 22.0 ± 11.9 | 19.7 ± 3 | 0.9 | |
| 258d | Br | 3 | 80.2 ± 8.8 | 234 ± 0.5 | 2.9 | |
| 258e | I | 3 | 119 ± 11 | 2200 ± 1250 | 18.5 | |
| 258f | H | 5 | 99.0 ± 28 | 550 ± 63 | 5.5 | |
| 259 | 616 ± 88 | 55200 ± 20000 | 89.3 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | Ki (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Uptake of [3H]DA) |
Selectivity uptake/binding |
|---|---|---|---|---|---|
 | |||||
| 260 (AHN 2-003) | H | 11.2 ± 11 | 9.7 | 0.9 | |
| 261a | 3-phenylpropyl | 41.9 ± 11 | 230 | 5.5 | |
| 261b | indole-3-ethyl | 44.6 ± 11 | 1200 | 26.9 | |
| 261c | 4-phenylbutyl | 8.51 ± 14 | 39 | 4.6 | |
| 261d | 4-(4′-nitrophenyl)butyl | 20.2 ± 11 | 650 | 32.2 | |
| 261e | 3-(4′-fluorophenyl)propyl | 60.7 ± 12 | - | - | |
| 262a | n-butyl | 24.6 ± 8 | 370 | 15.0 | |
| 262b | cyclopropylmethyl | 32.4 ± 9 | 180 | 5.5 | |
| 262c | allyl | 29.9 ± 10 | 14 | 0.5 | |
| 262d | benzyl | 82.2 ± 15 | 290 | 3.5 | |
| 262e | 4-fluorobenzyl | 95.6 ± 10 | 200 | 2.1 | |
| 262f | cinnanyl | 86.4 ± 12 | 180 | 2.1 | |
| 262g | [bis(4-fluorophenyl)methoxy]ethyl | 634 ± 23 | - | - | |
| 262h | [(4-nitrophenyl)phenylmethoxy]ethyl | 57.0 ± 17 | - | - | |
| 263 | acetyl | 2340 | 4600 | 2.0 | |
| 264 | formyl | 2020 ± 13 | 5400 | 2.7 | |
| 265a | Ts | 0%ɑ | - | - | |
| 265b | Ms | 18%ɑ | - | - | |
| (AHN 2-005)[42] | CH2CH=CH2 | - | - | - | |
| (JHW 007)[42] | CH2CH2CH2CH3 | - | - | - | |
| (GA 2-99)[42] | CH2CH2NH2 | - | - | - | |
| (GA 103)[42] | CH2CH2CH2CH2Ph | - | - | - | |
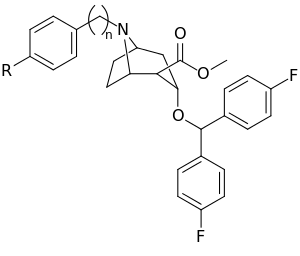
 | 266 | 108 ± 12 | 130 | 1.2 | |
ɑInhibition at 10 μM
| Compound | S. Singh's alphanumeric assignation (name) |
IC50 (nM) DAT (Binding of [3H]WIN 35428) |
IC50 (nM) 5-HTT (Binding of [3H]Citalopram) | |
|---|---|---|---|---|
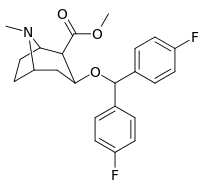 | R/S-268 | 2β,3β | >10000 | >1660 |
| R/S-269 | 2α,3β | 20300 | >1660 | |
| R/S-270 | 2α,3α | 22300 | >1660 | |
 | R/S-271 | 2β,3α | 520 | >1660 |
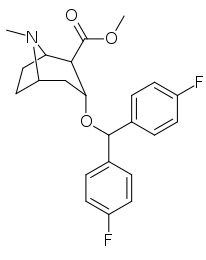
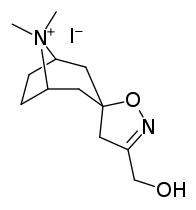
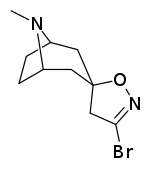
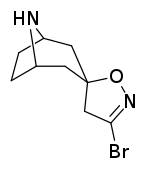
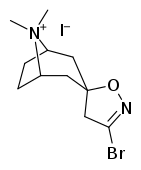
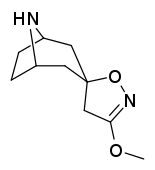
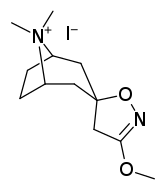
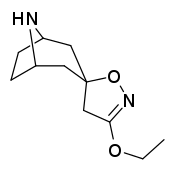
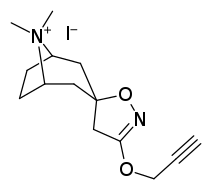
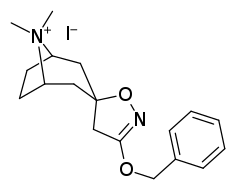
Tropanyl Isoxazoline Analogues
octane-3%2C5%E2%80%B2(4%E2%80%B2H)-isoxazole.svg.png)
octane-3%2C5%E2%80%B2(4%E2%80%B2H)-isoxazole.png)
This is the only known compound to allosterically modulate SERT in such a way within in vitro conditions (tianeptine has been shown to do similar, but has only shown efficacy doing so in living in vivo tissue samples). Considering its noncompetitive inhibition of 5-HT transporters decreasing Vmax with small change in the Km for serotonin, putatively stabilizing the cytoplasm-facing conformation of SERT: in such respect it is considered to have the opposite effect profile of the anti-addiction drug ibogaine (save for the function by which its anti-addictive properties are thought to be mediated, i.e. α3β4 nicotinic channel blockage. cf. 18-Methoxycornaridine for such nicotinergic activity without the likewise SERT affinity).[43]
Similarly, such peripheral DAT considerations (when, as often is, considered conformational rather than otherwise explained as being electrostatic) may constitute the difference in affinity, through allosertic occulsion, between cyclopentyl-ruthenium phenyltropane in its difference from the tricarbonyl-chromium
| Compound | Name | |||
|---|---|---|---|---|
| - |  |  |  |  |
| 4a | 4c | 5a | 5c | |
| - |  |  |  |  |
| 6a | 6b | 6c | 7a | |
| - |  |  |  |  |
| 7b | 7c | 8a | 8b | |
| - |  |  |  |  |
| 8c | 9a | 9b | 9c | |
| - |  |  |  | |
| 9c | 10a | 10b | 10c | |
| - |  |  |  |  |
| 11a | 11b | 12a | 12b |
8-Aminopentacyclo (σ receptor ligand) Trishomocubane Analogs
cf. other trishomocubanes such as basketane.
Sigma receptor agonists with nanomolar affinity such as CM156 have been shown to counteract the deleterious effects of cocaine when co-administered with it. Indicative that e.g. the local anesthetic effect at the sigma site mediating the toxicity or otherwise a cross over or tie in of cocaine's separate functionalities lowering threshold to its safety profile.[45]
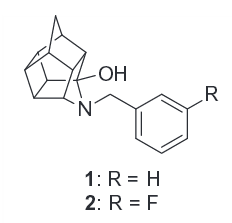
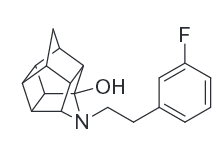



Polycyclic cage molecules: N-substituted 8-aminopentacyclo[5.4.0.02,6.03,10.05,9]undecanes (AHDs) & related.
The 3-FPh, 14b, has 1.2 ± 0.1 Ki (nM ± SEM) @ DAT.[46]
[[File:(1R,2S,3S,5S,6S,7R,8R,9S,10S)-N-((3-fluorophenyl)methyl)-N-methylpentacyclo(5.4.0.0<sup>2,6</sup>.0<sup>3,10</sup>.0<sup>5,9</sup>)undecan-8-amine.png]]
Dihydroimidazoles
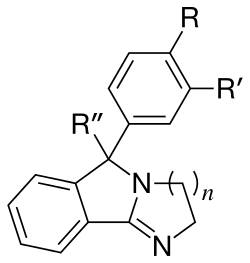
See: List of Mazindol analogues
Mazindol is usually considered a non-habituating (in humans, and some other mammals, but is habituating for e.g. Beagles[lower-alpha 44]) tetracyclic dopamine reuptake inhibitor (of somewhat spurious classification in the former).
It is a loosely functional analog used in cocaine research; due in large part to N-Ethylmaleimide being able to inhibit approximately 95% of the specific binding of [3H]Mazindol to the residues of the MAT binding site(s), however said effect of 10 mM N-Ethylmaleimide was prevented in its entirety by just 10 μM cocaine. Whereas neither 300 μM dopamine or D-amphetamine afforded sufficient protection to contrast the efficacy of cocaine.[lower-alpha 45]
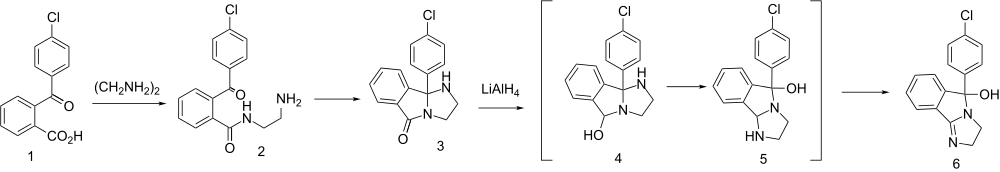
The above steps in its synthesis show the similitude of its precursors to the MAT reuptake inhibitor pipradrol & related compounds.
Local anesthetics (not usually CNS stimulants)
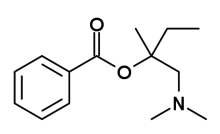
-2%2C2-dimethyl-1-phenylpropan-1-one.png)
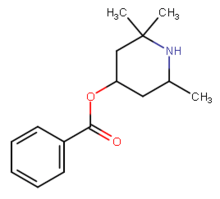
In animal studies, certain of the local anesthetics have displayed residual dopamine reuptake inhibitor properties,[48] although not normally ones that are easily available. These are expected to be more cardiotoxic than phenyltropanes. For example, dimethocaine has behavioral stimulant effects (and therefore not here listed below) if a dose of it is taken that is 10 times the amount of cocaine. Dimethocaine is equipotent to cocaine in terms of its anesthetic equivalency.[48] Intralipid "rescue" has been shown to reverse the cardiotoxic effects of sodium channel blockers and presumably those effects when from cocaine administered intravenously as well.
| Name | Other common names |
|---|---|
| Amylocaine | Stovaine |
| Articaine | Astracaine, Septanest, Septocaine, Ultracaine, Zorcaine |
| Benzocaine | |
| Bupivacaine | Marcaine, Sensorcaine, Vivacaine |
| Butacaine | |
| Carticaine | |
| Chloroprocaine | Nesacaine |
| Cinchocaine/Dibucaine | Cincain, Cinchocaine, Nupercainal, Nupercaine, Sovcaine |
| Cyclomethycaine | Surfacaine, Topocaine |
| Etidocaine | |
| Eucaine | α-eucaine, β-eucaine |
| Fomocaine[49] | |
| Fotocaine[49] | |
| Hexylcaine | Cyclaine, Osmocaine |
| Levobupivacaine | Chirocaine |
| Lidocaine/Lignocaine | Xylocaine, Betacaine |
| Mepivacaine | Carbocaine, Polocaine |
| Meprylcaine/Oracaine | Epirocain |
| Metabutoxycaine | Primacaine |
| Phenacaine/Holocaine | |
| Piperocaine | Metycaine |
| Pramocaine/Pramoxine | |
| Prilocaine | Citanest |
| Propoxycaine/Ravocaine | |
| Procaine/Novocaine | Borocaine (Procaine Borate), Ethocaine |
| Proparacaine/Alcaine | |
| Quinisocaine | Dimethisoquin |
| Risocaine | |
| Ropivacaine | Naropin |
| Tetracaine/Amethocaine | Pontocaine, Dicaine |
| Trimecaine | Mesdicain, Mesocain, Mesokain |
See also
Cocaine-N-oxide: 


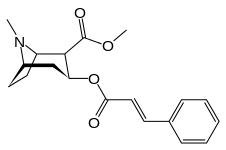
- Coca alkaloids, the ones relating to cocaine biosynthesis include: benzoylecgonine, ecgonidine, ecgonine, hydroxytropacocaine, methylecgonine cinnamate, tropacocaine & truxilline
- Cocaine metabolites (Human), which include: benzoylecgonine (BE), ecgonine methyl ester (EME), ecgonine, norcocaine, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE) & m-hydroxybenzoylecgonine
- Dopaminergics
- Federal Analog Act
- Pharmacophore
- Pharmacopoeia
- Pharmacokinetics
- Pharmacodynamics
Notes (inclu. specific locations of citations from within references used)
- [1] ←Page #969 (45th page of article) §III. ¶1. Final line. Last sentence.
- [1] ←Page #1,018 (94th page of article) 2nd column, 2nd paragraph.
- [1] ←Page #940 (16th page of article) underneath Table 8., above §4
- [1] ←Page #970 (46th page of article) Table 27. Figure 29.
- [1] ←Page #971 (47th page of article) Figure 30. & Page #973 (49th page of article) Table 28.
- [1] ←Page #982 (58th page of article)
- [1] ←Page #971 (47th page of article) Figure 30 & Page #971 (47th page of article) Figure 30 & Page #973 (49th page of article) Table 28
- [1] ←Page #972 (48th page of article) ¶2, Line 10.
- [1] ←Page #971 (47th page of article) Figure 30 & Page #971 (47th page of article) Figure 30 & Page #973 (49th page of article) Table 28
- [1] ←Page #971 (47th page of article) Figure 30 & Page #971 (47th page of article) Figure 30 & Page #973 (49th page of article) Table 28
- [1] ←Page #971 (47th page of article) Figure 30 & Page #971 (47th page of article) Figure 30 & Page #973 (49th page of article) Table 28
- [1] ←Page #974 (50st page of article) First (left) column, third ¶
- [1] ←Page #937 (13th page of article) Second (right) column, first ¶. Above/before §2
- [1] ←Page #974 (50th page of article) Final ¶ (5th), Second line.
- [1] ←Page #975 (51st page of article) First ¶, first line.
- [1] ←Page #975 (51st page of article) First ¶, 4th line.
- [1] ←Page #973 (49th page of article) §C. & Page #974 (50th page of article) Figure 31 & Page #976 (52nd page of article) Table 29.
- [1] ←Page #974 (50st page of article) First (left) column, fourth ¶
- [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- [1] ←Page #974 (50th page of article) Figure 31 & Page #977 (53rd page of article) Table 30.
- [1] ←Page #978 (54th page of article) §D & Page #980 (56th page of article) Figure 33 & Page #981 (57th page of article) Table 32.
- [1] ←Page #980 (56th page of article) Scheme 52.
- [1] ←Page #963 (39th page of article) 2nd (right side) column, 2nd paragraph.
- [1] ←Page #982 (58th page of article) §G & Page #983 (59th page of article) Figure 36 & Page #984 (60th page of article) Table 35.
- [1] ←Page #979 (55th page of article) Table 31.
- [1] ←Page #981 (57th page of article) §E & Page #982 (58th page of article) Table 33.
- [1] ←Page #970 (46th page of article) §B, 10th line
- [1] ←Page #971 (47th page of article) 1st ¶, 10th line
- [1] ←Page #949 (25th page of article) 3rd ¶, 20th line
- [1] ←Page #982 (58th page of article) 3rd ¶, lines 2, 5 & 6.
- [1] ←Page #982 (58th page of article) §F, Table 34 & Figure 35.
- [1] ←Page #984 (60th page of article) §H, Figure 37 & Page #985 (61st page of article) Table 36.
- [1] ←Page #984 (60th page of article) Scheme 56.
- [1] ←Page #986 (62nd page of article) §I, Table 37 & Scheme 58
- [1] ←Page #1,014 (90th page of article) §VIII, A. Figure 59.
- [1] ←Page #1,016 (92nd page of article) Figure 60.
- [1] ←Page #987 (63rd page of article) §IV, Figure 39 & Page #988 (64th page of article) Table 38.
- [1] ←Page #987 (63rd page of article) Figure 40, Page #988 (64th page of article) §B & Page #989 (65th page of article) Table 39.
- [1] ←Page #987 (63rd page of article) Figure 41, Page #989 (65th page of article) §C & Page #990 (66th page of article) Table 40.
- [1] ←Page #988 (64th page of article) Figure 42, Page #990 (66th page of article) §2 & Page #992 (68th page of article) Table 41.
- [1] ←Page #988 (64th page of article) Figure 43, Page #992 (68th page of article) §3 & Table 42.
- [1] ←Page #1,011 (87th page of article) §VII (7) 1st ¶.
- [1] ←Page #969 (45th page of article) 2nd (right-side) column 2nd ¶.
References
- Singh, Satendra; et al. (2000). "Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists" (PDF). Chem. Rev. 100 (3): 925–1024. doi:10.1021/cr9700538. PMID 11749256.
- Watson-Williams, E (1925). "Psicaine: An Artificial Cocaine". Br Med J. 1 (3340): 11. doi:10.1136/bmj.1.3340.11. PMC 2196615. PMID 20771843.
- Singh, S; Basmadjian, GP; Avor, K; Pouw, B; Seale, TW (1997). "A convenient synthesis of 2?- or 4?-hydroxycocaine". Synthetic Communications. 27 (22): 4003–4012. doi:10.1080/00397919708005923.
et. el-Moselhy, TF; Avor, KS; Basmadjian, GP (Sep 2001). "2?-substituted analogs of cocaine: synthesis and dopamine transporter binding potencies". Archiv der Pharmazie (Weinheim). 334 (8–9): 275–8. doi:10.1002/1521-4184(200109)334:8/9<275::aid-ardp275>3.0.co;2-b. PMID 11688137.
et. Seale, TW; Avor, K; Singh, S; Hall, N; Chan, HM; Basmadjian, GP (1997). "2?-Substitution of cocaine selectively enhances dopamine and norepinephrine transporter binding". NeuroReport. 8 (16): 3571–5. doi:10.1097/00001756-199711100-00030. PMID 9427328. - Smith, RM; Poquette, MA; Smith, PJ (1984). "Hydroxymethoxybenzoylmethylecgonines: New metabolites of cocaine from human urine". Journal of Analytical Toxicology. 8 (1): 29–34. doi:10.1093/jat/8.1.29. PMID 6708474.
- Gatley SJ, Yu DW, Fowler JS, MacGregor RR, Schlyer DJ, Dewey SL, Wolf AP, Martin T, Shea CE, Volkow ND (March 1994). "Studies with differentially labeled [11C]cocaine, [11C]norcocaine, [11C]benzoylecgonine, and [11C]- and 4′-[18F]fluorococaine to probe the extent to which [11C]cocaine metabolites contribute to PET images of the baboon brain". Journal of Neurochemistry. 62 (3): 1154–62. doi:10.1046/j.1471-4159.1994.62031154.x. PMID 8113802.
- Carroll, F. I.; Lewin, A. H.; Boja, J. W.; Kuhar, M. J. (1992). "Cocaine Receptor: Biochemical Characterization and Structure-Activity Relationships of Cocaine Analogues at Dopamine Transporter". Journal of Medicinal Chemistry. 35 (6): 969–981. doi:10.1021/jm00084a001. PMID 1552510.
- Seale, TW; Avor, K; Singh, S; Hall, N; Chan, HM; Basmadjian, GP (1997). "2′-Substitution of cocaine selectively enhances dopamine and norepinephrine transporter binding". NeuroReport. 8 (16): 3571–5. doi:10.1097/00001756-199711100-00030. PMID 9427328.
- Buckett, W. R.; Farquharson, Muriel E.; Haining, C. G. (1964). "The analgesic properties of some 14-substituted derivatives of codeine and codeinone". J. Pharm. Pharmacol. 16 (3): 174–182. doi:10.1111/j.2042-7158.1964.tb07440.x. PMID 14163981.
- Sakamuri, Sukumar; et al. (2000). "Synthesis of novel spirocyclic cocaine analogs using the Suzuki coupling". Tetrahedron Letters. 41 (13): 2055–2058. doi:10.1016/S0040-4039(00)00113-1.
- Isomura, Shigeki; Hoffman, Timothy Z.; Wirsching, Peter; Janda, Kim D. (2002). "Benzoylthio-. cocaine, analogue substitution. Synthesis, Properties, and Reactivity of Cocaine Benzoylthio Ester Possessing the Cocaine Absolute Configuration". J. Am. Chem. Soc. 124 (14): 3661–3668. doi:10.1021/ja012376y. PMID 11929256.
- Davis, Franklin A.; Gaddiraju, Narendra V.; Theddu, Naresh; Hummel, Joshua R.; Kondaveeti, Sandeep K.; Zdilla, Michael J. (2012). "Enantioselective Synthesis of Cocaine C-1 Analogues using Sulfinimines (N-Sulfinyl Imines)". The Journal of Organic Chemistry. 77 (5): 2345–2359. doi:10.1021/jo202652f. ISSN 0022-3263. PMID 22300308.
- Reith, M. E. A.; Ali, S.; Hashim, A.; Sheikh, I. S.; Theddu, N.; Gaddiraju, N. V.; Mehrotra, S.; Schmitt, K. C.; Murray, T. F.; Sershen, H.; Unterwald, E. M.; Davis, F. A. (2012). "Novel C-1 Substituted Cocaine Analogs Unlike Cocaine or Benztropine". Journal of Pharmacology and Experimental Therapeutics. 343 (2): 413–425. doi:10.1124/jpet.112.193771. ISSN 1521-0103. PMC 3477221. PMID 22895898. Full article
- Sharkey, J; Glen, KA; Wolfe, S; Kuhar, MJ (1988). "Cocaine binding at sigma receptors". Eur J Pharmacol. 149 (1–2): 171–4. doi:10.1016/0014-2999(88)90058-1. PMID 2840298.
- Nuwayhid, Samer J.; Werling, Linda L. (2006). "Sigma2 (σ2) receptors as a target for cocaine action in the rat striatum". European Journal of Pharmacology. 535 (1–3): 98–103. doi:10.1016/j.ejphar.2005.12.077. ISSN 0014-2999. PMID 16480713.
- Involvement of the Sigma1 Receptor in Cocaine-induced Conditioned Place Preference: Possible Dependence on Dopamine Uptake Blockade Pascal Romieu et al. Neuropsychopharmacology (2002) 26 444-455.10.1038/S0893-133X(01)00391-8
- Yoshihiro Hamaya, Hesham Abdelrazek, Gary R. Strichartz (2002). "A-854: Comparative Potency for Impulse-Blockade and for Cutaneous Analgesia of Traditional and Novel Local Anesthetics". Abstracts of American Society of Anesthesiologists Annual Meeting.
...hydroxypropylbenzoylecgonine (HPBE) is the only effective analgesic compound in [Esterom].
CS1 maint: multiple names: authors list (link) - U.S. Patent 6,479,509
- Kozikowski, A. P.; Simoni, D.; Roberti, M.; Rondanin, R.; Wang, S.; Du, P.; Johnson, K. M. (1999). "Synthesis of 8-oxa analogues of norcocaine endowed with interesting cocaine-like activity". Bioorganic & Medicinal Chemistry Letters. 9 (13): 1831–1836. doi:10.1016/S0960-894X(99)00273-5. PMID 10406650.
- Hoepping, Alexander (2000). "Novel Conformationally Constrained Tropane Analogues by 6- e ndo-trig Radical Cyclization and Stille Coupling − Switch of Activity toward the Serotonin and/or Norepinephrine Transporter". Journal of Medicinal Chemistry. 43 (10): 2064–2071. doi:10.1021/jm0001121. PMID 10821718.
- Zhang, Ao (2002). "Thiophene derivatives: a new series of potent norepinephrine and serotonin reuptake inhibitors". Bioorganic. 12 (7): 993–995. doi:10.1016/S0960-894X(02)00103-8. PMID 11909701.
- Zhang, Ao (2002). "Further Studies on Conformationally Constrained Tricyclic Tropane Analogues and Their Uptake Inhibition at Monoamine Transporter Sites: Synthesis of ( Z )-9-(Substituted arylmethylene)-7-azatricyclo[4.3.1.0 3,7 ]decanes as a Novel Class of Serotonin Transporter Inhibitors". Journal of Medicinal Chemistry. 45 (9): 1930–1941. doi:10.1021/jm0105373. PMID 11960503.
- Davies, HM; Saikali, E; Sexton, T; Childers, SR (1993). "Novel 2-substituted cocaine analogs: binding properties at dopamine transport sites in rat striatum". Eur. J. Pharmacol. 244 (1): 93–7. doi:10.1016/0922-4106(93)90063-f. PMID 8420793.
- "Drugbank website "drug card", "(DB00907)" for Cocaine: Giving ten targets of the molecule in vivo, including dopamine/serotonin sodium channel affinity & K-opioid affinity". Drugbank.ca. Retrieved 9 March 2010.
- Sahlholm, Kristoffer; Nilsson, Johanna; Marcellino, Daniel; Fuxe, Kjell; Århem, Peter (2012). "Voltage sensitivities and deactivation kinetics of histamine H3 and H4 receptors". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1818 (12): 3081–3089. doi:10.1016/j.bbamem.2012.07.027. PMID 22885137. ...Agonist potency at some neurotransmitter receptors has been shown to be regulated by voltage, a mechanism which has been suggested to play a crucial role in the regulation of neurotransmitter release by inhibitory autoreceptors...
- Enantioselective synthesis of strobamine and its analogues Xing Zhang et al. Center for Organic and Medicinal Chemistry, Research Triangle Institute. Issue in Honor of Prof. James M.Cook ARKIVOC 2010 (iv)96-103
- The Alkaloids; Vol. 44, Geoffrey Cordell
- Appell, Michael; Dunn, William J.; Reith, Maarten E.A.; Miller, Larry; Flippen-Anderson, Judith L. (2002). "An Analysis of the Binding of Cocaine Analogues to the Monoamine Transporters Using Tensor Decomposition 3-D QSAR". Bioorganic & Medicinal Chemistry. 10 (5): 1197–1206. doi:10.1016/S0968-0896(01)00389-3. ISSN 0968-0896. PMID 11886784.
- Hicks, MJ; De, BP; Rosenberg, JB; Davidson, JT; Moreno, AY; Janda, KD; Wee, S; Koob, GF; Hackett, NR; Kaminsky, SM; Worgall, S; Toth, M; Mezey, JG; Crystal, RG (2011). "Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs". Mol Ther. 19 (3): 612–9. doi:10.1038/mt.2010.280. PMC 3048190. PMID 21206484.
- Kinsey, BM; Kosten, TR; Orson, FM (2010). "Active immunotherapy for the Treatment of Cocaine Dependence". Drugs of the Future. 35 (4): 301–306. doi:10.1358/dof.2010.035.04.1474292. PMC 3142961. PMID 21796226.
- Wee, S; Hicks, MJ; De, BP; Rosenberg, JB; Moreno, AY; Kaminsky, SM; Janda, KD; Crystal, RG; Koob, GF (2011). "Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects". Neuropsychopharmacology. 37 (5): 1083–91. doi:10.1038/npp.2011.200. PMC 3306868. PMID 21918504.
- Catalytic antibodies against cocaine and methods of using and producing same Google patents US 6566084 B1
- Deng, Shixian; Bharat, Narine; de Prada, Paloma; Landry, Donald W. (2004). "Substrate-assisted antibody catalysis". Organic & Biomolecular Chemistry. 2 (3): 288–90. doi:10.1039/b314264g. ISSN 1477-0520. PMID 14747854.
- Ho, M; Segre, M (2003). "Inhibition of cocaine binding to the human dopamine transporter by a single chain anti-idiotypic antibody: its cloning, expression, and functional properties". Biochim Biophys Acta. 1638 (3): 257–66. doi:10.1016/s0925-4439(03)00091-7. PMC 3295240. PMID 12878327.
- Schabacker, DS; Kirschbaum, KS; Segre, M (2000). "Exploring the feasibility of an anti-idiotypic cocaine vaccine: analysis of the specificity of anticocaine antibodies (Ab1) capable of inducing Ab2beta anti-idiotypic antibodies". Immunology. 100 (1): 48–56. doi:10.1046/j.1365-2567.2000.00004.x. PMC 2326984. PMID 10809958.
- Zhou, Jia; He, Rong; Johnson, Kenneth M.; Ye, Yanping; Kozikowski, Alan P. (2004). "Piperidine-Based Nocaine/Modafinil Hybrid Ligands as Highly Potent Monoamine Transporter Inhibitors: Efficient Drug Discovery by Rational Lead Hybridization". Journal of Medicinal Chemistry. 47 (24): 5821–5824. doi:10.1021/jm040117o. ISSN 0022-2623. PMC 1395211. PMID 15537337.
- Skeptics Stack Exchange: Is sugar one element away from cocaine (or any other drug?)
- Velázquez-Sánchez, Clara; García-Verdugo, José M.; Murga, Juan; Canales, Juan J. (2013). "The atypical dopamine transport inhibitor, JHW 007, prevents amphetamine-induced sensitization and synaptic reorganization within the nucleus accumbens". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 44: 73–80. doi:10.1016/j.pnpbp.2013.01.016. ISSN 0278-5846. PMID 23385166.
- Tanda, G; Newman, A; Ebbs, AL; Tronci, V; Green, J; Tallarida, RJ; Katz, JL (2009). "Combinations of Cocaine with other Dopamine Uptake Inhibitors: Assessment of Additivity". J Pharmacol Exp Ther. 330 (3): 802–9. doi:10.1124/jpet.109.154302. PMC 2729796. PMID 19483071.
- Schmitt, KC; Rothman, RB; Reith, ME (2013). "Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates". J. Pharmacol. Exp. Ther. 346 (1): 2–10. doi:10.1124/jpet.111.191056. PMC 3684841. PMID 23568856.
- Rothman, RB; Baumann, MH; Prisinzano, TE; Newman, AH (2008). "Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction". Biochem Pharmacol. 75 (1): 2–16. doi:10.1016/j.bcp.2007.08.007. PMC 2225585. PMID 17897630.
- Runyon, SP; Carroll, FI (2006). "Dopamine transporter ligands: recent developments and therapeutic potential". Curr Top Med Chem. 6 (17): 1825–43. doi:10.2174/156802606778249775. PMID 17017960.
- Loland, C. J.; Desai, R. I.; Zou, M.-F.; Cao, J.; Grundt, P.; Gerstbrein, K.; Sitte, H. H.; Newman, A. H.; Katz, J. L.; Gether, U. (2007). "Relationship between Conformational Changes in the Dopamine Transporter and Cocaine-Like Subjective Effects of Uptake Inhibitors". Molecular Pharmacology. 73 (3): 813–823. doi:10.1124/mol.107.039800. ISSN 0026-895X. PMID 17978168.
- Dallanoce, Clelia; Canovi, Mara; Matera, Carlo; Mennini, Tiziana; De Amici, Marco; Gobbi, Marco; De Micheli, Carlo (2012). "A novel spirocyclic tropanyl-Δ2-isoxazoline derivative enhances citalopram and paroxetine binding to serotonin transporters as well as serotonin uptake". Bioorganic & Medicinal Chemistry. 20 (21): 6344–6355. doi:10.1016/j.bmc.2012.09.004. ISSN 0968-0896. PMID 23022052.
- C. Dallanoce et al. - Bioorg. Med. Chem. 20 (2012) 6344-6355
- Xu, Y. T.; Kaushal, N.; Shaikh, J.; Wilson, L. L.; Mesangeau, C.; McCurdy, C. R.; Matsumoto, R. R. (2010). "A Novel Substituted Piperazine, CM156, Attenuates the Stimulant and Toxic Effects of Cocaine in Mice". Journal of Pharmacology and Experimental Therapeutics. 333 (2): 491–500. doi:10.1124/jpet.109.161398. ISSN 0022-3565. PMC 2872963. PMID 20100904.
- Banister, Samuel D.; Manoli, Miral; Barron, Melissa L.; Werry, Eryn L.; Kassiou, Michael (2013). "N-substituted 8-aminopentacyclo[5.4.0.02,6.03,10.05,9]undecanes as σ receptor ligands with potential neuroprotective effects". Bioorganic & Medicinal Chemistry. 21 (19): 6038–6052. doi:10.1016/j.bmc.2013.07.045. ISSN 0968-0896. PMID 23981939.
- Ruetsch, YA; Böni, T; Borgeat, A (Aug 2001). "From cocaine to ropivacaine: the history of local anesthetic drugs". Curr Top Med Chem. 1 (3): 175–82. doi:10.2174/1568026013395335. PMID 11895133.
- Wilcox, K.M.; Kimmel, H.L.; Lindsey, K.P.; Votaw, J.R.; Goodman, M.M.; Howell, L.L. (2005). "In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys" (PDF). Synapse. 58 (4): 220–228. CiteSeerX 10.1.1.327.1264. doi:10.1002/syn.20199. PMID 16206183. Archived from the original (PDF) on 2010-06-11.
- Schoenberger, Matthias; Damijonaitis, Arunas; Zhang, Zinan; Nagel, Daniel; Trauner, Dirk (2014). "Development of a New Photochromic Ion Channel Blocker via Azologization of Fomocaine". ACS Chemical Neuroscience. 5 (7): 514–518. doi:10.1021/cn500070w. ISSN 1948-7193. PMC 4102962. PMID 24856540. nih.gov article
- U.S. Patent 6,479,509 Patent inventor Frank Ivy Carroll, Assignee: Research Triangle Institute
- U.S. patent US6479509 B1 structures given for submission, 5th compound down in image.
External links
| Wikimedia Commons has media related to Cocaine analogs. |
- U. S. Provisional Patent Application listing examples of compounds which are tropanes for prospective use in research
- Article on cocaine analogue research
- List of tropanyl type molecules and their CAS Registry Numbers
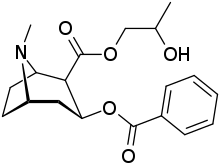

-6-methyl-6-azabicyclo(3.2.1)octan-3-yl_benzoate.svg.png)
-6-methyl-6-azabicyclo(3.2.1)octan-3-yl_benzoate.svg.png)