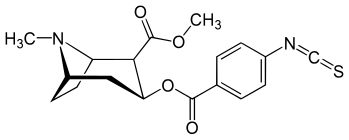
(R)-p-Isothiocyanatobenzoylecgonine methyl ester
(R)-p-Isothiocyanatobenzoylecgonine methyl ester (p-ISOCOC) is a cocaine analogue and irreversible (covalent) binding inhibitor of the cocaine receptor, as well as irreversible blocker of dopamine uptake by DAT (the latter being unlike its C3 homologue m-Isococ). p-Isococ also blocks the high-affinity cocaine site in preference to the low-affinity site.[1]
 | |
| Names | |
|---|---|
| IUPAC name
methyl (1S,3S,4R,5R)-3-(4-isothiocyanatobenzoyl)oxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate | |
| Other names
p-ISOCOC, p-Isococ | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C18H20N2O4S | |
| Molar mass | 360.427 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- RTI-76, covalent binding phenyltropane
- 4'-Fluorococaine
References
- Carroll FI, Lewin AH, Boja JW, Kuhar MJ (March 1992). "Cocaine receptor: biochemical characterization and structure-activity relationships of cocaine analogues at the dopamine transporter". Journal of Medicinal Chemistry. 35 (6): 969–81. doi:10.1021/jm00084a001. PMID 1552510.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.