Solriamfetol
Solriamfetol, sold under the brand name Sunosi, is a medication used for the treatment of excessive sleepiness associated with narcolepsy and sleep apnea.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Sunosi |
| Other names | SKL-N05, ADX-N05, ARL-N05, YKP10A, R228060, and JZP-110; (R)-2-amino-3-phenylpropylcarbamate hydrochloride |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619040 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~95% |
| Protein binding | 13.3–19.4% |
| Metabolism | negligible |
| Elimination half-life | ~7.1 h |
| Excretion | urine (95% unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
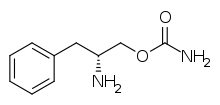
| Formula | C10H14N2O2 |
| Molar mass | 194.234 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects include headache, nausea, anxiety, and trouble sleeping.[1] It is a norepinephrine–dopamine reuptake inhibitor (NDRI). It is derived from d-phenylalanine and its chemical name is (R)-2-amino-3-phenylpropylcarbamate hydrochloride.[2]
The drug was discovered by a subsidiary of SK Group, which licensed rights outside of 11 countries in Asia to Aerial Pharma in 2011.[3]
History
The drug was discovered by a subsidiary of SK Group, which licensed rights outside of 11 countries in Asia to Aerial Pharma in 2011.[3] Aerial ran two Phase II trials of the drug in narcolepsy[4] before selling the license to solriamfetol to Jazz in 2014; Jazz Pharmaceuticals paid Aerial $125 million up front and will pay Aerial and SK up to $272 million in milestone payments, and will pay double-digit royalties to SK.[3][5]
In 2019, solriamfetol was approved in the United States to improve wakefulness in adults with narcolepsy or obstructive sleep apnea (OSA).[6][7] It was granted orphan drug designation.[8]
The U.S. Food and Drug Administration (FDA) approved solriamfetol based primarily on evidence from five clinical trials (Trial 1/NCT02348593, Trial 2/NCT02348606, Trial 3/NCT02348619, Trial 4/NCT02348632, Trial 5 NCT01681121) of 622 patients with narcolepsy or obstructive sleep apnea (OSA).[6] The trials were conducted in Canada, Europe, and the United States.[6]
Names
During development it has been called SKL-N05, ADX-N05, ARL-N05, and JZP-110.[9]
References
- "Sunosi- solriamfetol tablet, film coated". DailyMed. 16 October 2019. Retrieved 24 November 2019.
- Abad VC, Guilleminault C (2017). "New developments in the management of narcolepsy". Nature and Science of Sleep. 9: 39–57. doi:10.2147/NSS.S103467. PMC 5344488. PMID 28424564.
- Ji-young S (5 March 2018). "SK Biopharmaceuticals' narcolepsy drug on track to hitting US market". The Korea Herald.
- Sullivan SS, Guilleminault C (2015). "Emerging drugs for common conditions of sleepiness: obstructive sleep apnea and narcolepsy". Expert Opinion on Emerging Drugs. 20 (4): 571–82. doi:10.1517/14728214.2015.1115480. PMID 26558298.
- Garde D (14 January 2014). "Jazz bets up to $397M on Aerial's narcolepsy drug". FierceBiotech.
- "Drug Trials Snapshots: Sunosi". U.S. Food and Drug Administration (FDA). 16 April 2019. Archived from the original on 28 September 2019. Retrieved 24 November 2019.

- "Drug Approval Package: Sunosi". U.S. Food and Drug Administration (FDA). 29 April 2019. Retrieved 24 November 2019.

- "Solriamfetol Orphan Drug Approval". U.S. Food and Drug Administration (FDA). Retrieved 24 November 2019.

- "Solriamfetol - Jazz Pharmaceuticals/SK Biopharmaceuticals". AdisInsight. Retrieved 15 April 2018.
External links
- "Solriamfetol". Drug Information Portal. U.S. National Library of Medicine.