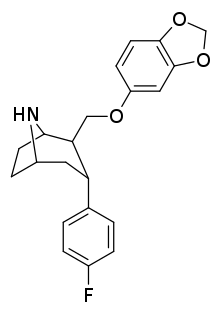
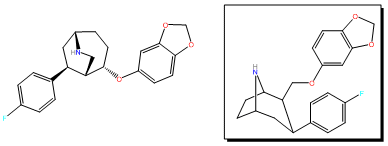
RTI-274
RTI(-4229)-274, or 2β-((3,4-Methyl
 | |
| Identifiers | |
|---|---|
| |
| Chemical and physical data | |
| Formula | C21H22FNO3 |
| Molar mass | 355.409 g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Introduction
Very few esters of phenyltropanes are actually known to have been reported.
NS2330 and NS2359 both have α,β stereochemistry.
NS2214 appears to have been abandoned now, RTI-336 was their latest compound.
RTI decided that they wanted to make all 8 stereoisomers of the phenyltropane paroxetine homolog.[1]
| MAT IC50 (nM) Nor/tropane-Paroxetine Hybrids | ||||
| Compound | [3H]CFT | [3H]Paroxetine | [3H]Nisoxetine | |
| Paroxetine | ? → 623 | ? → 0.28 | ? → 535 | |
| R | "β,β" | 308 → 835 | 294 → 480 | 5,300 → 37,400 |
| α,β | 172 → 142 | 52.9 → 90 | 26,600 → 2,500 | |
| β,α | 3.01 → 3.86 | 422 → 5.62 | 123 → 14.4 | |
| S | "β,β" | 1,050 → 1,210 | 88.1 → 424 | 27,600 → 17,300 |
| α,β | 1,500 → 27.6 | 447→ 55.8 | 2,916 → 1,690 | |
| β,α | 298 → 407 | 178 → 19 | 12,400 → 1,990 | |
- N-demethylating the S-α,β (1S,2S,3R) isomer resulted in a 54-fold increase in DAT IC50.
In the case of nocaine it is understood that the SR enantiomer is the one that should be demethylated if it is wanted to improve DAT affinity.
That is the same enantiomer that is used in the production of paroxetine.
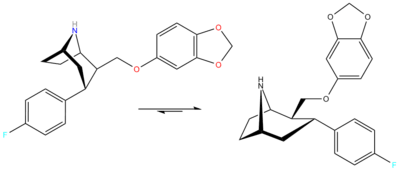
Skeletal rearrangement
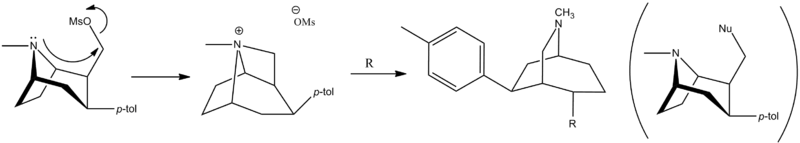
Four years later some unrelated authors cited a skeletal rearrangement accounts for this.[2] Diagram
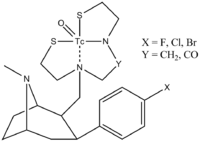
Notice that they are not only interested in ethers, but nitrogen containing Nu's ("TRODAT")[3]
The metal is called "Technetium" and is bound by a chelating agent.
The authors state that at first the acid is halogenated, the amide is prepared, and reduced.
Erratum
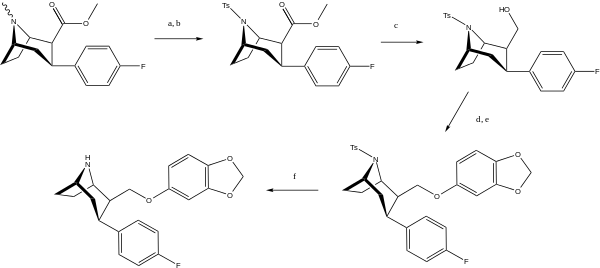
(a) (1) 1-chloroethyl chloroformate, 1,2-dichloroethane, reflux; (2) MeOH reflux; (b) p-toluenesulfonyl chloride, triethylamine; (c) LiAlH4, THF, rt; (d) trifluoromethanesulfonic anhydride, pyridine, CH2Cl2; (e) Na, sesamol, THF; (f) 5% Na/Hg amalgam, Na2HPO4, MeOH.
| MAT IC50 (Ki) N-Methyl → De-methyl | |||
| Compound | [3H]CFT | [3H]Nisoxetine | [3H]paroxetine |
| R-β,β | ? → 3 | ? → 2 (0.2) | ? → 6 (4) |
| S-β,β | ? → ? | ? → ? (?) | ? → ? (?) |
| R-"nonane" | 308 → 835 | 294 (27) → 480 (44) | 5,300 (3200) → 37,400 (22,500) |
| S-"nonane" | 1050 → 1210 | 88 (8) → 424 (39) | 27,600 (16,600) → 17,300 (10,400) |
To solve the problem of the unexpected aza-bicyclo[3.2.2]nonane rearrangement product, the original synthesis had to be modified as follows;[4] WIN 35428 was N-demethylated and then the NH amine was reacted with a suitable protecting group so that N is no longer nucleophilic. In their case they used a tosyl.[3]
See also
References
- Keverline-Frantz KI, Boja JW, Kuhar MJ, Abraham P, Burgess JP, Lewin AH, Carroll FI (January 1998). "Synthesis and ligand binding of tropane ring analogues of paroxetine". Journal of Medicinal Chemistry. 41 (2): 247–57. doi:10.1021/jm970669p. PMID 9457247.
- Ogier L, Turpin F, Baldwin RM, Riché F, Law H, Innis RB, Tamagnan G (May 2002). "Rearrangement of a mesylate tropane intermediate in nucleophilic substitution reactions. Synthesis of aza-bicyclo[3.2.1]octane and aza-bicyclo[3.2.2]nonane ethers, imides, and amines". The Journal of Organic Chemistry. 67 (11): 3637–42. doi:10.1021/jo010973x. PMID 12027674.
- Singh S (March 2000). "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews. 100 (3): 925–1024. doi:10.1021/cr9700538. PMID 11749256. S2CID 36764655.
- Runyon SP, Burgess JP, Abraham P, Keverline-Frantz KI, Flippen-Anderson J, Deschamps J, et al. (April 2005). "Synthesis, structural identification, and ligand binding of tropane ring analogs of paroxetine and an unexpected aza-bicyclo[3.2.2]nonane rearrangement product". Bioorganic & Medicinal Chemistry. 13 (7): 2439–49. doi:10.1016/j.bmc.2005.01.046. PMID 15755646.