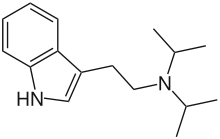
Diisopropyltryptamine
Diisopropyltryptamine (/ˌdaɪˌaɪsoʊˌproʊpɪlˈtrɪptəmiːn/; also known as N,N-diisopropyltryptamine or DiPT) is a psychedelic hallucinogenic drug of the tryptamine family that has a unique effect. While the majority of hallucinogens affect the visual sense, DiPT is primarily aural.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | N,N-Diisopropyltryptamine, DiPT |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H24N2 |
| Molar mass | 244.382 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hallucinogenic properties
DiPT's effects are primarily aural. At lower doses, Alexander Shulgin reported effects similar to a flanging or a phase shift. At medium and higher dosages, the effect of DiPT is typically a radical shift downward in perceived pitch. This shift tends to be nonlinear, in that the shift downwards varies in relation to the initial pitch. This can produce bizarre sounds and render music disharmonious.[1] There has been an experiment involving subjects with perfect pitch, the goal of which was to determine whether the pitch difference is truly distortive or linear, the results of which indicated that there is no clear relationship between perceived pitch and actual pitch.[1] Aside from these, the most prevalent non-auditory effect is inner ear pressure (which has been painful in some instances, for example when combined with MDMA).[1]
Chemistry
DiPT is a derivative of tryptamine formed by substituting isopropyl groups for the two hydrogen atoms attached to the non-aromatic nitrogen atom in the tryptamine molecule.
Pharmacodynamics
| Binding sites | Binding affinity (Ki, μM)[2] |
|---|---|
| 5-HT1A | 0.538 |
| 5-HT2A | 1.2 |
| 5-HT2C | 6.5 |
| D1 | >25 |
| D2 | >25 |
| D3 | >25 |
| α1A | >12 |
| α2A | 3.6 |
| TAAR1 | >15 |
| H1 | 0.92 |
| SERT | 0.18 |
| DAT | 4.1 |
| NET | 8.9 |
Legal status
United Kingdom
As is the case with many PiHKAL and TiHKAL drugs, it is Class A in the UK, making it illegal to possess or use.
United States
DiPT is not scheduled at the federal level in the United States,[3] but it could be considered an analog of 5-MeO-DiPT, in which case purchase, sale, or possession could be prosecuted under the Federal Analog Act. Some of the people arrested in Operation Web Tryp were selling DiPT.
Florida
"DiPT (N,N-Diisopropyltryptamine)" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[4]
Sweden
Sweden's public health agency suggested classifying DiPT as a hazardous substance, on May 15, 2019.[5]
References
- Shulgin A (1997). TiHKAL: Tryptamines I Have Known and Loved. Berkeley, CA USA: Transform Press. pp. 403–406. ISBN 0-9630096-9-9.
- Rickli A, Moning OD, Hoener MC, Liechti ME (August 2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). European Neuropsychopharmacology. 26 (8): 1327–37. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487.
- "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I." Drug Enforcement Administration (DEA). U.S. Department Of Justice.
- "Chapter 893 - Drug Abuse Prevention and Control". Florida Statutes.
- "Folkhälsomyndigheten föreslår att 20 ämnen klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 15 May 2019.