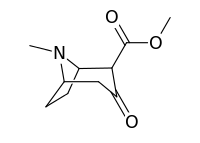
2-Carbomethoxytropinone
2-Carbomethoxytropinone (2-CMT) is a commonly used organic intermediate in the synthesis of cocaine and its analogues.[1] As of at least 1999 no reaction pathway has been discovered that synthesizes cocaine-like compounds without utilizing the reduction of 2-CMT.[2] The structure of cocaine was discovered by Richard Willstätter in 1898 after he synthesized it from 2-carbomethoxytropinone.[3][4] Although it was originally believed that 2-CMT in nature was ultimately derived from ornithine and acetic acid,[5] subsequent research has indicated other pathways exist for the biosynthesis of 2-CMT.[6][7] A β-keto ester, 2-CMT exists in equilibrium with its keto–enol tautomer.
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (1S,4R,5R)-8-methyl-3-oxo-8-azabicyclo[3.2.1]octane-4-carboxylate | |
| Other names
Carbomethoxy-tropinone; 2-Carbomethoxy-3-tropinone; Carbmethoxy-tropinone; Methyl 8-methyl-3-oxobicyclo[3.2.1]octane-2-carboxylate; Methyl 8-methyl-3-oxo-8-azabicyclo[3.2.1]octane-4-carboxylate; 2-(Methoxycarbonyl)-3-tropanone | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H15NO3 | |
| Molar mass | 197.234 g·mol−1 |
| Melting point | 104 °C (219 °F; 377 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
2-CMT (3) can be synthesized from 1,3-acetonedicarboxylate anhydride (1) by methanolysis followed by reaction with methylamine and succinaldehyde.[8]
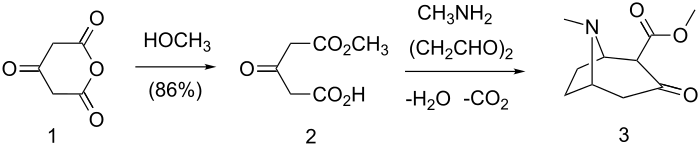 Synthesis of 2-CMT
Synthesis of 2-CMT
See also
References
- Findlay, Stephen P. (1957). "Concerning 2-Carbomethoxytropinone*". The Journal of Organic Chemistry. 22 (11): 1385. doi:10.1021/jo01362a022.
- Simoni, Daniele; Roberti, Marinella; Andrisano, Vincenza; Manferdini, Monica; Rondanin, Riccardo; Invidiata, Francesco Paolo (1999). "Two-carbon bridge substituted cocaines: Enantioselective synthesis, attribution of the absolute configuration and biological activity of novel 6- and 7-methoxylated cocaines". Il Farmaco. 54 (5): 275–87. doi:10.1016/S0014-827X(99)00027-0. PMID 10418122.
- Humphrey, Andrew J.; O'Hagan, David (2001). "Tropane alkaloid biosynthesis. A century old problem unresolved". Natural Product Reports. 18 (5): 494–502. doi:10.1039/b001713m. PMID 11699882.
- Findlay, Stephen P. (1954). "The Three-dimensional Structure of the Cocaines. Part I. Cocaine and Pseudococaine". Journal of the American Chemical Society. 76 (11): 2855. doi:10.1021/ja01640a001.
- Leete, Edward (1983). "Chemistry of the tropane alkaloids. 33. 2-Carbomethoxy-3-tropinone: An advanced intermediate in the biosynthesis of cocaine". Journal of the American Chemical Society. 105 (22): 6727. doi:10.1021/ja00360a038.
- Leete, Edward.; Kim, Sung Hoon. (1988). "A revision of the generally accepted hypothesis for the biosynthesis of the tropane moiety of cocaine". Journal of the American Chemical Society. 110 (9): 2976. doi:10.1021/ja00217a051.
- Jirschitzka, Jan; Dolke, Franziska; d’Auria, John C. (2013). "Increasing the Pace of New Discoveries in Tropane Alkaloid Biosynthesis". New Light on Alkaloid Biosynthesis and Future Prospects. Advances in Botanical Research. 68. p. 39. doi:10.1016/B978-0-12-408061-4.00002-X. ISBN 9780124080614.
- Proc F. according to Findlay