Nerve growth factor IB
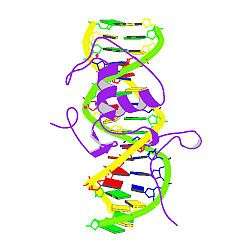
The nerve growth factor IB (NGFIB) also known as Nur77 or NR4A1 (nuclear receptor subfamily 4 group A member 1) is a protein that in humans is encoded by the NR4A1 gene.[5][6]
Nerve growth factor IB is a member of the Nur nuclear receptor family[7] of intracellular transcription factors.[6][8] NGFIB is involved in cell cycle mediation, inflammation and apoptosis.[9]
The NGFIB protein plays a key role in mediating inflammatory responses in macrophages.[9] In addition, subcellular localization of the NGFIB protein appears to play a key role in the survival and death of cells.[10]
Expression is induced by phytohemagglutinin in human lymphocytes and by serum stimulation of arrested fibroblasts. Translocation of the protein from the nucleus to mitochondria induces apoptosis. Multiple alternatively spliced variants, encoding the same protein, have been identified.[5]
Structure
The NR4A1 gene contains seven exons. An amino terminal transactivation domain is encoded in exon 2, a DNA-binding domain in exons 3 and 4, and dimerisation and ligand-binding domains is exons 5 to 7.[11]
The protein has an atypical ligand-binding domain that is unlike the classical ligand-binding domain in most nuclear receptors. The classical domain contains a ligand-receiving pocket and co-activator site, both of which are lacking in the NR4A family. Whereas most nuclear receptors have a hydrophobic surface that results in a cleft, NGFI-B has a hydrophilic surface.[7]
Cofactors interact with NGFI-B at a hydrophobic region between helices 11 and 12 to modulate transcription.[7]
Function
Along with the two other Nur family members, NGFIB is expressed in macrophages following inflammatory stimuli. This process is mediated by the NF-κB (nuclear factor-kappa B) complex, a ubiquitous transcription factor involved in cellular response to stress.[9]
NGFIB can be induced by many physiological and physical stimuli. These include physiological stimuli such as "fatty acids, stress, prostaglandins, growth factors, calcium, inflammatory cytokines, peptide hormones, phorbol esters, and neurotransmitters" and physical stimuli including "magnetic fields, mechanical agitation (causing fluid shear stress), and membrane depolarization".[7] Ligands do not bind to NGFIB, so modulation occurs at the level of protein expression and posttranslational modification.[9] Besides these, NR4A1 can mediate T cell function, the transcription factor NR4A1 is stably expressed at high levels in tolerant T cells. Overexpression of NR4A1 inhibits effector T cell differentiation, whereas deletion of NR4A1 overcomes T cell tolerance and exaggerates effector function, as well as enhancing immunity against tumor and chronic virus. Mechanistically, NR4A1 is preferentially recruited to binding sites of the transcription factor AP-1, where it represses effector gene expression by inhibiting AP-1 function. NR4A1 binding also promotes acetylation of histone 3 at lysine 27 (H3K27ac), leading to activation of tolerance-related genes.[12]
Biochemistry
Nerve growth factor IB binds as a monomer or homodimer to response element NBRE[13] and as a homodimer to NurRE.[14] It is also capable of heterodimerising with COUP-TF (an orphan nuclear receptor) and retinoid X receptor (RXR) in mediating transcription in response to retinoids.[15]
The binding sites on the response elements for NGFI-B, which are common to the two other members of the Nur family, are:[7]
- NBRE - 5’-A/TAAAGGTCA,
- NurRE - a AAAT(G/A)(C/T)CA repeat,
- RXR - DX, a motif.
Evolution and homology
Nerve growth factor IB has the systematic HUGO gene symbol NR4A1. It belongs to a group of three closely related orphan receptors, the Nur family, which has the symbol NR4A. The other two members are nuclear receptor related 1 protein (denoted by symbol NR4A2) and neuron-derived orphan receptor 1 (NR4A3).
NGFIB has a high degree of structural similarity with other family members at the DNA-binding domain with 91-95% sequence conservation. The C-terminal ligand-binding domain is conserved to a lesser extent at 60% and the N-terminal AB region is not conserved, differing in each member.[7]
The three members are similar in biochemistry and function. They are immediate early genes activated in a ligand-independent manner that bind at the same sites on response elements.[11]
NGFIB and the rest of the Nur family are structurally similar to other nuclear receptor superfamily members, but contain an extra intron. The DNA-binding domain at exons 3 and 4 of the NR4A1 gene is conserved among all members of the nuclear receptor.[11]
NR4A1 has homologous genes in a range of species including neuronal growth factor-induced clone B in rats, Nur77 in mice and TR3 in humans.[16]
Pathology
Along with 16 other genes, Nerve growth factor IB is a signature gene in the metastasis of some primary solid tumours. It is downregulated in this process.[17]
Interactions
Nerve growth factor IB has been shown to interact with:
- AKT1,[18]
- Bcl-2,[19]
- HIF1A,[20]
- Nuclear receptor co-repressor 2,[21]
- Promyelocytic leukemia protein,[22]
- Retinoid X receptor alpha,[19] and
- Von Hippel-Lindau tumor suppressor.[20]
References
- GRCh38: Ensembl release 89: ENSG00000123358 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000023034 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: NR4A1 nuclear receptor subfamily 4, group A, member 1".
- Chang C, Kokontis J, Liao SS, Chang Y (1989). "Isolation and characterization of human TR3 receptor: a member of steroid receptor superfamily". J. Steroid Biochem. 34 (1–6): 391–5. doi:10.1016/0022-4731(89)90114-3. PMID 2626032.
- Maxwell MA, Muscat GE (2006). "The NR4A subgroup: immediate early response genes with pleiotropic physiological roles". Nucl Recept Signal. 4: e002. doi:10.1621/nrs.04002. PMC 1402209. PMID 16604165.
- Milbrandt J (1988). "Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene". Neuron. 1 (3): 183–8. doi:10.1016/0896-6273(88)90138-9. PMID 3272167.
- Pei L, Castrillo A, Tontonoz P (2006). "Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77". Mol. Endocrinol. 20 (4): 786–94. doi:10.1210/me.2005-0331. PMID 16339277.
- Zhang XK (2007). "Targeting Nur77 translocation". Expert Opin. Ther. Targets. 11 (1): 69–79. doi:10.1517/14728222.11.1.69. PMID 17150035.
- Saucedo-Cardenas O, Kardon R, Ediger TR, Lydon JP, Conneely OM (1997). "Cloning and structural organization of the gene encoding the murine nuclear receptor transcription factor, NURR1". Gene. 187 (1): 135–9. doi:10.1016/S0378-1119(96)00736-6. PMID 9073077.
- Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, et al. (March 2019). "Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction". Nature. 567 (7749): 525–529. doi:10.1038/s41586-019-0979-8. PMC 6507425. PMID 30814730.
- Hiromura M, Suizu F, Narita M, Kinowaki K, Noguchi M (2006). "Identification of nerve growth factor-responsive element of the TCL1 promoter as a novel negative regulatory element". J. Biol. Chem. 281 (38): 27753–64. doi:10.1074/jbc.M602420200. PMID 16835233.
- Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J (1997). "Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells". Mol. Cell. Biol. 17 (10): 5946–51. doi:10.1128/MCB.17.10.5946. PMC 232442. PMID 9315652.
- Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, Reed JC, Dawson MI, Zhang XK (2006). "Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt". Oncogene. 25 (21): 2974–86. doi:10.1038/sj.onc.1209358. PMID 16434970.
- Wei T, Geiser AG, Qian HR, Su C, Helvering LM, Kulkarini NH, Shou J, N'Cho M, Bryant HU, Onyia JE (2007). "DNA microarray data integration by ortholog gene analysis reveals potential molecular mechanisms of estrogen-dependent growth of human uterine fibroids". BMC Women's Health. 7: 5. doi:10.1186/1472-6874-7-5. PMC 1852551. PMID 17407572.
- Ramaswamy S, Ross KN, Lander ES, Golub TR (2003). "A molecular signature of metastasis in primary solid tumors". Nat. Genet. 33 (1): 49–54. doi:10.1038/ng1060. PMID 12469122.
- Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce CM (March 2001). "Akt phosphorylates and regulates the orphan nuclear receptor Nur77". Proc. Natl. Acad. Sci. U.S.A. 98 (7): 3690–4. doi:10.1073/pnas.051003198. PMC 31113. PMID 11274386.
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK (February 2004). "Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3". Cell. 116 (4): 527–40. doi:10.1016/S0092-8674(04)00162-X. PMID 14980220.
- Kim BY, Kim H, Cho EJ, Youn HD (February 2008). "Nur77 upregulates HIF-α by inhibiting pVHL-mediated degradation". Experimental & Molecular Medicine. 40 (1): 71–83. doi:10.3858/emm.2008.40.1.71. PMC 2679322. PMID 18305400.
- Sohn YC, Kwak E, Na Y, Lee JW, Lee SK (November 2001). "Silencing mediator of retinoid and thyroid hormone receptors and activating signal cointegrator-2 as transcriptional coregulators of the orphan nuclear receptor Nur77". J. Biol. Chem. 276 (47): 43734–9. doi:10.1074/jbc.M107208200. PMID 11559707.
- Wu WS, Xu ZX, Ran R, Meng F, Chang KS (May 2002). "Promyelocytic leukemia protein PML inhibits Nur77-mediated transcription through specific functional interactions". Oncogene. 21 (24): 3925–33. doi:10.1038/sj.onc.1205491. PMID 12032831.
Further reading
- Winoto A, Littman DR (2002). "Nuclear hormone receptors in T lymphocytes". Cell. 109 Suppl (2): S57–66. doi:10.1016/S0092-8674(02)00710-9. PMID 11983153.
- Engelse MA, Arkenbout EK, Pannekoek H, de Vries CJ (2004). "Activin and TR3 orphan receptor: two 'atheroprotective' genes as evidenced in dedicated mouse models". Clin. Exp. Pharmacol. Physiol. 30 (11): 894–9. doi:10.1046/j.1440-1681.2003.03928.x. PMID 14678255.
- Bondy GP (1991). "Phorbol ester, forskolin, and serum induction of a human colon nuclear hormone receptor gene related to the NUR 77/NGFI-B genes". Cell Growth Differ. 2 (4): 203–8. PMID 1651101.
- Nakai A, Kartha S, Sakurai A, Toback FG, DeGroot LJ (1991). "A human early response gene homologous to murine nur77 and rat NGFI-B, and related to the nuclear receptor superfamily". Mol. Endocrinol. 4 (10): 1438–43. doi:10.1210/mend-4-10-1438. PMID 2283997.
- Ryseck RP, Macdonald-Bravo H, Mattéi MG, Ruppert S, Bravo R (1989). "Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor". EMBO J. 8 (11): 3327–35. doi:10.1002/j.1460-2075.1989.tb08494.x. PMC 401469. PMID 2555161.
- Uemura H, Mizokami A, Chang C (1995). "Identification of a new enhancer in the promoter region of human TR3 orphan receptor gene. A member of steroid receptor superfamily". J. Biol. Chem. 270 (10): 5427–33. doi:10.1074/jbc.270.10.5427. PMID 7890657.
- Hirata Y, Kiuchi K, Chen HC, Milbrandt J, Guroff G (1993). "The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B". J. Biol. Chem. 268 (33): 24808–12. PMID 8227042.
- Harrison DC, Roberts J, Campbell CA, Crook B, Davis R, Deen K, Meakin J, Michalovich D, Price J, Stammers M, Maycox PR (2000). "TR3 death receptor expression in the normal and ischaemic brain". Neuroscience. 96 (1): 147–60. doi:10.1016/S0306-4522(99)00502-3. PMID 10777386.
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X (2000). "Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3". Science. 289 (5482): 1159–64. doi:10.1126/science.289.5482.1159. PMID 10947977.
- Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce CM (2001). "Akt phosphorylates and regulates the orphan nuclear receptor Nur77". Proc. Natl. Acad. Sci. U.S.A. 98 (7): 3690–4. doi:10.1073/pnas.051003198. PMC 31113. PMID 11274386.
- Sohn YC, Kwak E, Na Y, Lee JW, Lee SK (2001). "Silencing mediator of retinoid and thyroid hormone receptors and activating signal cointegrator-2 as transcriptional coregulators of the orphan nuclear receptor Nur77". J. Biol. Chem. 276 (47): 43734–9. doi:10.1074/jbc.M107208200. PMID 11559707.
- Lee MO, Kang HJ, Cho H, Shin EC, Park JH, Kim SJ (2001). "Hepatitis B virus X protein induced expression of the Nur77 gene". Biochem. Biophys. Res. Commun. 288 (5): 1162–8. doi:10.1006/bbrc.2001.5910. PMID 11700033.
- Slagsvold HH, Østvold AC, Fallgren AB, Paulsen RE (2002). "Nuclear receptor and apoptosis initiator NGFI-B is a substrate for kinase ERK2". Biochem. Biophys. Res. Commun. 291 (5): 1146–50. doi:10.1006/bbrc.2002.6579. PMID 11883936.
- Wu WS, Xu ZX, Ran R, Meng F, Chang KS (2002). "Promyelocytic leukemia protein PML inhibits Nur77-mediated transcription through specific functional interactions". Oncogene. 21 (24): 3925–33. doi:10.1038/sj.onc.1205491. PMID 12032831.
- Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ (2002). "Induction of apoptosis by TPA and VP-16 is through translocation of TR3". World J. Gastroenterol. 8 (3): 446–50. doi:10.3748/wjg.v8.i3.446. PMC 4656418. PMID 12046067.
- Wansa KD, Harris JM, Muscat GE (2002). "The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment". J. Biol. Chem. 277 (36): 33001–11. doi:10.1074/jbc.M203572200. PMID 12082103.
- Chtarbova S, Nimmrich I, Erdmann S, Herter P, Renner M, Kitajewski J, Müller O (2002). "Murine Nr4a1 and Herpud1 are up-regulated by Wnt-1, but the homologous human genes are independent from beta-catenin activation". Biochem. J. 367 (Pt 3): 723–8. doi:10.1042/BJ20020699. PMC 1222938. PMID 12153396.
- Wu Q, Liu S, Ye XF, Huang ZW, Su WJ (2002). "Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells". Carcinogenesis. 23 (10): 1583–92. doi:10.1093/carcin/23.10.1583. PMID 12376465.
External links
| Wikimedia Commons has media related to NR4A1. |
- orphan+nuclear+receptor+NGFI-B at the US National Library of Medicine Medical Subject Headings (MeSH)
- orphan+nuclear+receptor+TR3 at the US National Library of Medicine Medical Subject Headings (MeSH)