Chloroacetamide
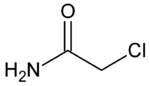
Chloroacetamide (2-chloroacetamide) is a chlorinated organic compound with the molecular formula CHCl2CONH2. It is used as an herbicide[1] and a preservative.[2] It is a colorless (older samples appear yellow) crystalline substance with characteristic smell, readily soluble in water.[3]
 | |
| Names | |
|---|---|
| IUPAC name
2-Chloroacetamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.068 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H4ClNO | |
| Molar mass | 93.51 g·mol−1 |
| Appearance | Colorless or yellow crystals |
| Melting point | 120 °C (248 °F; 393 K) |
| 90 g/L at 25°C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
It is produced by ammonolysis of the methyl ester:[4]
- ClCH2CO2CH3 + NH3 → ClCH2C(O)NH2 + CH3OH
Hazards
Chloroacetamide is toxic, irritates eyes and skin, and may cause an allergic reaction. It is suspected of reproductive toxicity and teratogenicity.[5] It decomposes when heated above 225 °C and creates toxic gases including chlorine and nitrogen oxides.[3]
gollark: What do you want to say exactly?
gollark: It'd make sense.
gollark: Maybe things older than a year are dropped off?
gollark: We must kill ~~all~~ <1-year-old golds. **FOR THE RATIOS!**
gollark: Okay, probably not.
See also
References
- Herbicides - Epochem Archived 2008-05-20 at the Wayback Machine
- Acetamide, 2-chloro- - Government of Canada Archived 2009-01-31 at the Wayback Machine
- 2-CHLOROACETAMIDE
- Koenig, G.; Lohmar, E.; Rupprich, N. "Chloroacetic Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_537.CS1 maint: multiple names: authors list (link)
- "DECISION du 14 juin 2012" (PDF) (in French). Agence Nationale de Sécurité du Médicament et des Produits de Santé. Cite journal requires
|journal=(help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.