3C-BZ
3C-BZ (4-benzyloxy-3,5-dimethoxyamphetamine) is a lesser-known psychedelic drug and a substituted amphetamine. 3C-BZ was first synthesized by Alexander Shulgin. In his book PiHKAL, the dosage range is listed as 25–200 mg and the duration as 18–24 hours.[1] According to anecdotal reports from the substance's entry in PiHKAL, 3C-BZ's effects can vary significantly, ranging from intensified emotions and strange dreams, to effects similar to those of LSD or TMA.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of 3C-BZ.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
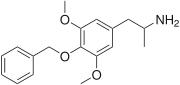
1-(4-benzyloxy-3,5-methoxyphenyl)propan-2-amine | |
| Other names
4-Benzyloxy-3,5-methoxyamphetamine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H23NO3 | |
| Molar mass | 301.386 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
3C-BZ was originally synthesized by Alexander Shulgin starting from 5-methoxyeugenol (4-allyl-2,6-dimethoxyphenol) through a reaction with benzyl chloride to form the benzyloxy derivative of 5-methoxyeugenol. The obtained benzyl derivative was reacted with tetranitromethane to form 1-[4-(Benzyloxy)-3,5-dimethoxyphenyl]-2-nitro-1-propene, from which 3C-BZ is obtained by reduction of the nitropropene with lithium aluminum hydride. Another possible synthetic route would be the reaction of benzyl chloride with syringaldehyde to form 3,5-dimethoxy-4-benzyloxybenzaldehyde followed by condensation with nitroethane to form 1-[4-(Benzyloxy)-3,5-dimethoxyphenyl]-2-nitro-1-propene. The obtained nitropropene can be reduced using lithium aluminum hydride, Red-Al, or an aluminum-mercury amalgam.
References
- Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. 3C-BZ Entry in PiHKAL
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||