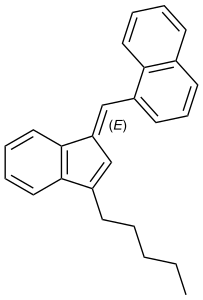
JWH-176
JWH-176 is an analgesic drug which acts as a cannabinoid receptor agonist. Its binding affinity at the CB1 receptor is 26.0 nM, making it more potent than THC itself,[1] however JWH-176 is particularly notable in that it is a hydrocarbon containing no heteroatoms. This demonstrates that reasonably high-affinity cannabinoid binding and agonist effects can be produced by compounds with no hydrogen bonding capacity at all, relying merely on Van der Waals interactions to bind to the receptor.[2] It was discovered by, and named after, John W. Huffman.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H24 |
| Molar mass | 324.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Stereochemistry
JWH-176 is the (E)-stereoisomer of 1-([3-pentylinden-1-ylidine]methyl)naphthalene, whereas JWH-171 is the mixture of the (E)- and (Z)-isomers.[3]
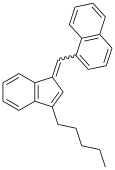 JWH-171
JWH-171
Legal status
In the United States, CB1 receptor agonists of the 1-(1-naphthylmethylene)indene class such as JWH-176 and JWH-171 are Schedule I Controlled Substances.[4]
As of 23rd December 2009, any compound structurally derived from 1–(1–naphthylmethyl)indene by substitution at the 3–position of the indene ring by an alkyl group is a Class B drug in the United Kingdom. [5]
See also
References
- Huffman JW, Padgett LW (2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
- Pertwee RG. "Cannabinoids". Handbook of Experimental Pharmacology. 168. Springer. p. 269. ISBN 3-540-22565-X.
- Huffman JW, Padgett LW (2010-12-10). "Recent Developments in the Medicinal Chemistry of Cannabimimetic Indoles, Pyrroles and Indenes". In Rahman A, Iqbal Choudhary M, Reitz AB (eds.). Frontiers in Medicinal Chemistry. 4. pp. 661–687 (681). doi:10.2174/978160805207310904010661. ISBN 978-1-60805-346-9.
- : Schedules of controlled substances
- "The Misuse of Drugs Act 1971 (Amendment) Order 2009", legislation.gov.uk, The National Archives, SI 2009/3209