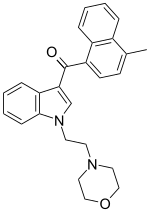
JWH-193
JWH-193 is a drug from the aminoalkylindole and naphthoylindole families which acts as a cannabinoid receptor agonist. It was invented by the pharmaceutical company Sanofi-Winthrop in the early 1990s. JWH-193 has a binding affinity at the CB1 receptor of 6 nM, binding around seven times more tightly than the parent compound JWH-200,[1] though with closer to twice the potency of JWH-200 in activity tests.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H26N2O2 |
| Molar mass | 398.506 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as JWH-193 are Schedule I Controlled Substances.[2]
Related compounds
A structural isomer of JWH-193 with the methyl group on the indole ring instead of the naphthoyl ring, was also found to be of similarly increased potency over JWH-200.[3][4]
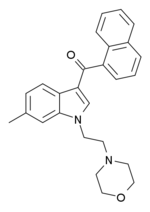
6-Methyl-JWH-200
gollark: Just carry the laptop. I don't see the problem. If you have the keyboard you probably have a bag of some sort.
gollark: But I only use it for very quick scripting and SSHing into my server for fixes, because æ.
gollark: I have heard of it and use it.
gollark: Why are you doing programming on Android? Stop it.
gollark: This caused problems on my server, which is set to UTC, but *not* on my laptop, which is local time.
References
- Huffman JW, Padgett LW (2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
- : Schedules of controlled substances
- Eissenstat MA, Bell MR, D'Ambra TE, Alexander EJ, Daum SJ, Ackerman JH, et al. (August 1995). "Aminoalkylindoles: structure-activity relationships of novel cannabinoid mimetics". Journal of Medicinal Chemistry. 38 (16): 3094–105. doi:10.1021/jm00016a013. PMID 7636873.
- Shim JY, Collantes ER, Welsh WJ, Subramaniam B, Howlett AC, Eissenstat MA, Ward SJ (November 1998). "Three-dimensional quantitative structure-activity relationship study of the cannabimimetic (aminoalkyl)indoles using comparative molecular field analysis". Journal of Medicinal Chemistry. 41 (23): 4521–32. doi:10.1021/jm980305c. PMID 9804691.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.