Fenoprofen
Fenoprofen is a nonsteroidal anti-inflammatory drug (NSAID). Fenoprofen calcium is used for symptomatic relief for rheumatoid arthritis, osteoarthritis, and mild to moderate pain. Fenoprofen is marketed in the US as Nalfon.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681026 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Major urinary metabolites are fenoprofen glucuronide and 4′-hydroxyfenoprofen glucuronide. |
| Elimination half-life | 3 hours |
| Excretion | Renal (~90%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.045.231 |
| Chemical and physical data | |
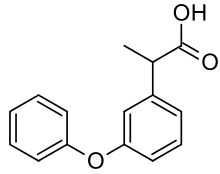
| Formula | C15H14O3 |
| Molar mass | 242.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
As of 2015 the cost for a typical month of medication in the United States is 50 to US$100.[1]
Pharmacology
Decreases inflammation, pain, and fever, probably through inhibition of cyclooxygenase (COX-2 inhibitor) activity and prostaglandin synthesis.
Contraindications
History of significantly impaired renal function; patients with known hypersensitivity to any component of the product; patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs; treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
Drug interactions
- Aminoglycosides (e.g. gentamicin): Plasma aminoglycoside levels may be elevated.
- Angiotensin-converting enzyme (ACE) inhibitors: Antihypertensive effect of ACE inhibitors may be diminished.
- Anticoagulants: Coadministration may prolong prothrombin time.
- Aspirin: Fenoprofen Cl may be increased; coadministration is not recommended.
- Diuretics: Patients treated with fenoprofen may be resistant to the effects of loop diuretics and thiazides.
- Hydantoins, sulfonamides, sulfonylureas: Fenoprofen may displace these drugs from their binding site.
- Lithium: Renal Cl of lithium may be reduced and plasma levels may be elevated, which may increase the risk of lithium toxicity.
- Methotrexate: May increase methotrexate levels.
- Phenobarbital: May decrease fenoprofen t ½ . Dosage adjustments of fenoprofen may be required if phenobarbital is added or withdrawn.
- SSRIs (e.g. fluoxetine, citalopram): The risk of GI effects may be increased.
Laboratory test interactions
False elevation in free and total serum T 3 as measured by Amerlex-M kit.
Brand names
- UK - Fenopron (Typharm Limited)
References
- Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 8X. ISBN 9781284057560.